Caveolin expression changes in the neurovascular unit after juvenile traumatic brain injury: signs of blood-brain barrier healing?
- PMID: 25450954
- PMCID: PMC4431593
- DOI: 10.1016/j.neuroscience.2014.10.035
Caveolin expression changes in the neurovascular unit after juvenile traumatic brain injury: signs of blood-brain barrier healing?
Abstract
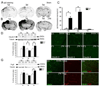
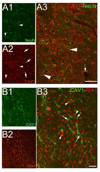
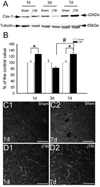
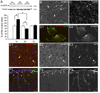
Traumatic brain injury (TBI) is one of the major causes of death and disability in pediatrics, and results in a complex cascade of events including the disruption of the blood-brain barrier (BBB). A controlled-cortical impact on post-natal 17-day-old rats induced BBB disruption by IgG extravasation from 1 to 3 days after injury and returned to normal at day 7. In parallel, we characterized the expression of three caveolin isoforms, caveolin 1 (cav-1), caveolin 2 (cav-2) and caveolin 3 (cav-3). While cav-1 and cav-2 are expressed on endothelial cells, both cav-1 and cav-3 were found to be present on reactive astrocytes, in vivo and in vitro. Following TBI, cav-1 expression was increased in blood vessels at 1 and 7 days in the perilesional cortex. An increase of vascular cav-2 expression was observed 7 days after TBI. In contrast, astrocytic cav-3 expression decreased 3 and 7 days after TBI. Activation of endothelial nitric oxide synthase (eNOS) (via its phosphorylation) was detected 1 day after TBI and phospho-eNOS was detected both in association with blood vessels and with astrocytes. The molecular changes involving caveolins occurring in endothelial cells following juvenile-TBI might participate, independently of eNOS activation, to a mechanism of BBB repair while, they might subserve other undefined roles in astrocytes.
Keywords: astrocyte; blood–brain barrier; caveolin; endothelium; juvenile traumatic brain injury.
Copyright © 2014 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest
Figures



References
-
- Armstead WM. Cerebral hemodynamics after traumatic brain injury of immature brain. Exp Toxicol Pathol. 1999;51:137–142. - PubMed
-
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

