Spinally projecting preproglucagon axons preferentially innervate sympathetic preganglionic neurons
- PMID: 25450967
- PMCID: PMC4300405
- DOI: 10.1016/j.neuroscience.2014.10.043
Spinally projecting preproglucagon axons preferentially innervate sympathetic preganglionic neurons
Abstract
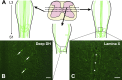
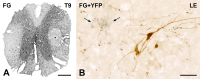
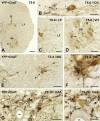
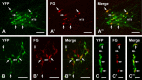
Glucagon-like peptide-1 (GLP-1) affects central autonomic neurons, including those controlling the cardiovascular system, thermogenesis, and energy balance. Preproglucagon (PPG) neurons, located mainly in the nucleus tractus solitarius (NTS) and medullary reticular formation, produce GLP-1. In transgenic mice expressing glucagon promoter-driven yellow fluorescent protein (YFP), these brainstem PPG neurons project to many central autonomic regions where GLP-1 receptors are expressed. The spinal cord also contains GLP-1 receptor mRNA but the distribution of spinal PPG axons is unknown. Here, we used two-color immunoperoxidase labeling to examine PPG innervation of spinal segments T1-S4 in YFP-PPG mice. Immunoreactivity for YFP identified spinal PPG axons and perikarya. We classified spinal neurons receiving PPG input by immunoreactivity for choline acetyltransferase (ChAT), nitric oxide synthase (NOS) and/or Fluorogold (FG) retrogradely transported from the peritoneal cavity. FG microinjected at T9 defined cell bodies that supplied spinal PPG innervation. The deep dorsal horn of lower lumbar cord contained YFP-immunoreactive neurons. Non-varicose, YFP-immunoreactive axons were prominent in the lateral funiculus, ventral white commissure and around the ventral median fissure. In T1-L2, varicose, YFP-containing axons closely apposed many ChAT-immunoreactive sympathetic preganglionic neurons (SPN) in the intermediolateral cell column (IML) and dorsal lamina X. In the sacral parasympathetic nucleus, about 10% of ChAT-immunoreactive preganglionic neurons received YFP appositions, as did occasional ChAT-positive motor neurons throughout the rostrocaudal extent of the ventral horn. YFP appositions also occurred on NOS-immunoreactive spinal interneurons and on spinal YFP-immunoreactive neurons. Injecting FG at T9 retrogradely labeled many YFP-PPG cell bodies in the medulla but none of the spinal YFP-immunoreactive neurons. These results show that brainstem PPG neurons innervate spinal autonomic and somatic motor neurons. The distributions of spinal PPG axons and spinal GLP-1 receptors correlate well. SPN receive the densest PPG innervation. Brainstem PPG neurons could directly modulate sympathetic outflow through their spinal inputs to SPN or interneurons.
Keywords: choline acetyltransferase; glucagon-like peptide-1; green fluorescent protein; nucleus of the solitary tract; parasympathetic preganglionic neurons; retrograde tracing.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures






References
-
- Anderson C.R., Edwards S.L. Intraperitoneal injections of Fluorogold reliably labels all sympathetic preganglionic neurons in the rat. J Neurosci Methods. 1994;53:137–141. - PubMed
-
- Buse J.B., Rosenstock J., Sesti G., Schmidt W.E., Montanya E., Brett J.H., Zychma M., Blonde L. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

