Hydroxylated tropolones inhibit hepatitis B virus replication by blocking viral ribonuclease H activity
- PMID: 25451058
- PMCID: PMC4335860
- DOI: 10.1128/AAC.04617-14
Hydroxylated tropolones inhibit hepatitis B virus replication by blocking viral ribonuclease H activity
Abstract
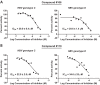
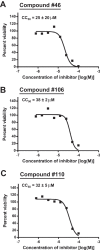
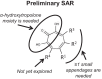
Hepatitis B virus (HBV) remains a major human pathogen despite the development of both antiviral drugs and a vaccine, in part because the current therapies do not suppress HBV replication far enough to eradicate the virus. Here, we screened 51 troponoid compounds for their ability to suppress HBV RNaseH activity and HBV replication based on the activities of α-hydroxytropolones against HIV RNaseH, with the goal of determining whether the tropolone pharmacophore may be a promising scaffold for anti-HBV drug development. Thirteen compounds inhibited HBV RNaseH, with the best 50% inhibitory concentration (IC50) being 2.3 μM. Similar inhibition patterns were observed against HBV genotype D and C RNaseHs, implying limited genotype specificity. Six of 10 compounds tested against HBV replication in culture suppressed replication via blocking of viral RNaseH activity, with the best 50% effective concentration (EC50) being 0.34 μM. Eighteen compounds inhibited recombinant human RNaseH1, and moderate cytotoxicity was observed for all compounds (50% cytotoxic concentration [CC50]=25 to 79 μM). Therapeutic indexes ranged from 3.8 to 94. Efficient inhibition required an intact α-hydroxytropolone moiety plus one or more short appendages on the tropolone ring, but a wide variety of constituents were permissible. These data indicate that troponoids and specifically α-hydroxytropolones are promising lead candidates for development as anti-HBV drugs, providing that toxicity can be minimized. Potential anti-RNaseH drugs are envisioned to be employed in combination with the existing nucleos(t)ide analogs to suppress HBV replication far enough to block genomic maintenance, with the goal of eradicating infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E, European Concerted Action on Viral Hepatitis (EUROHEP) . 2002. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol 97:2886–2895. doi:10.1111/j.1572-0241.2002.07057.x. - DOI - PubMed
-
- Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, Kern ER, McHugh JA, Petersen GM, Rein MF, Strader DB, Trotter HT. 2009. National Institutes of Health Consensus Development Conference Statement: management of hepatitis B. Ann Intern Med 150:104–110. doi:10.7326/0003-4819-150-2-200901200-00100. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

