A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs
- PMID: 25451075
- PMCID: PMC7113785
- DOI: 10.1016/j.antiviral.2014.11.010
A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs
Abstract
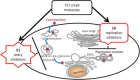
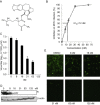
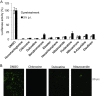
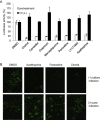
With the recent emergence of Middle East Respiratory Syndrome coronavirus in humans and the outbreak of devastating porcine epidemic diarrhea coronavirus in swine, therapeutic intervention is urgently needed. However, anti-coronavirus drugs currently are not available. In an effort to assist rapid development of anti-coronavirus drugs, here we screened the NIH Clinical Collection in cell culture using a luciferase reporter-expressing recombinant murine coronavirus. Of the 727 compounds screened, 84 were found to have a significant anti-coronavirus effect. Further experiments revealed that 51 compounds blocked virus entry while 19 others inhibited viral replication. Additional validation studies with the top 3 inhibitors (hexachlorophene, nitazoxanide and homoharringtonine) demonstrated robust anti-coronavirus activities (a reduction of 6 to 8log10 in virus titer) with an IC50 ranging from 11nM to 1.2μM. Furthermore, homoharringtonine and hexachlorophene exhibited broad antiviral activity against diverse species of human and animal coronaviruses. Since the NIH Clinical Collection consists of compounds that have already been through clinical trials, these small molecule inhibitors have a great potential for rapid development as anti-coronavirus drugs.
Keywords: Coronavirus; NCC library; Screening; Small molecule.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures




References
-
- Aulner N., Danckaert A., Rouault-Hardoin E., Desrivot J., Helynck O., Commere P.H., Munier-Lehmann H., Spath G.F., Shorte S.L., Milon G., Prina E. High content analysis of primary macrophages hosting proliferating Leishmania amastigotes: application to anti-leishmanial drug discovery. PLoS Negl. Trop. Dis. 2013;7:E2154. - PMC - PubMed
-
- Barlough J.E., Shacklett B.L. Antiviral studies of feline infectious peritonitis virus in vitro. Vet. Rec. 1994;135:177–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

