Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila
- PMID: 25451195
- PMCID: PMC4365050
- DOI: 10.1016/j.neuron.2014.09.032
Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila
Abstract
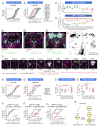
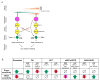
An organism's behavioral decisions often depend upon the relative strength of appetitive and aversive sensory stimuli, the relative sensitivity to which can be modified by internal states like hunger. However, whether sensitivity to such opposing influences is modulated in a unidirectional or bidirectional manner is not clear. Starved flies exhibit increased sugar and decreased bitter sensitivity. It is widely believed that only sugar sensitivity changes, and that this masks bitter sensitivity. Here we use gene- and circuit-level manipulations to show that sweet and bitter sensitivity are independently and reciprocally regulated by starvation in Drosophila. We identify orthogonal neuromodulatory cascades that oppositely control peripheral taste sensitivity for each modality. Moreover, these pathways are recruited at increasing hunger levels, such that low-risk changes (higher sugar sensitivity) precede high-risk changes (lower sensitivity to potentially toxic resources). In this way, state-intensity-dependent, reciprocal regulation of appetitive and aversive peripheral gustatory sensitivity permits flexible, adaptive feeding decisions.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
-
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Current biology: CB. 2000;10:187–194. - PubMed
-
- Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

