Heterotypic gap junctions at glutamatergic mixed synapses are abundant in goldfish brain
- PMID: 25451276
- PMCID: PMC4282965
- DOI: 10.1016/j.neuroscience.2014.10.057
Heterotypic gap junctions at glutamatergic mixed synapses are abundant in goldfish brain
Abstract
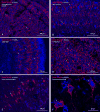
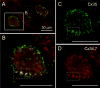
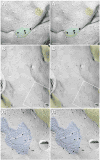
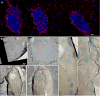
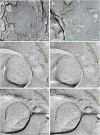
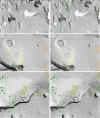
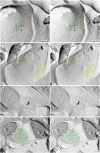
Gap junctions provide for direct intercellular electrical and metabolic coupling. The abundance of gap junctions at "large myelinated club ending (LMCE)" synapses on Mauthner cells (M-cells) of the teleost brain provided a convenient model to correlate anatomical and physiological properties of electrical synapses. There, presynaptic action potentials were found to evoke short-latency electrical "pre-potentials" immediately preceding their accompanying glutamate-induced depolarizations, making these the first unambiguously identified "mixed" (i.e., chemical plus electrical) synapses in the vertebrate CNS. We recently showed that gap junctions at these synapses exhibit asymmetric electrical resistance (i.e., electrical rectification), which we correlated with total molecular asymmetry of connexin composition in their apposing gap junction hemiplaques, with connexin35 (Cx35) restricted to axon terminal hemiplaques and connexin34.7 (Cx34.7) restricted to apposing M-cell plasma membranes. We now show that similarly heterotypic neuronal gap junctions are abundant throughout goldfish brain, with labeling exclusively for Cx35 in presynaptic hemiplaques and exclusively for Cx34.7 in postsynaptic hemiplaques. Moreover, the vast majority of these asymmetric gap junctions occur at glutamatergic axon terminals. The widespread distribution of heterotypic gap junctions at glutamatergic mixed synapses throughout goldfish brain and spinal cord implies that pre- vs. postsynaptic asymmetry at electrical synapses evolved early in the chordate lineage. We propose that the advantages of the molecular and functional asymmetry of connexins at electrical synapses that are so prominently expressed in the teleost CNS are unlikely to have been abandoned in higher vertebrates. However, to create asymmetric coupling in mammals, where most gap junctions are composed of connexin36 (Cx36) on both sides, would require some other mechanism, such as differential phosphorylation of connexins on opposite sides of the same gap junction or on asymmetric differences in the complement of their scaffolding and regulatory proteins.
Keywords: connexin34.7; connexin35; freeze-fracture replica immunogold labeling (FRIL); gap junctions; immunofluorescence microscopy.
Copyright © 2014 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures






References
-
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Postlethwait JH. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

