Phospholamban interactome in cardiac contractility and survival: A new vision of an old friend
- PMID: 25451386
- PMCID: PMC4312245
- DOI: 10.1016/j.yjmcc.2014.10.005
Phospholamban interactome in cardiac contractility and survival: A new vision of an old friend
Abstract
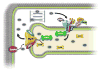
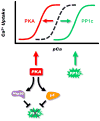
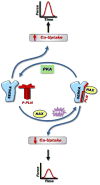
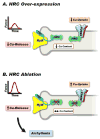
Depressed sarcoplasmic reticulum (SR) calcium cycling, reflecting impaired SR Ca-transport and Ca-release, is a key and universal characteristic of human and experimental heart failure. These SR processes are regulated by multimeric protein complexes, including protein kinases and phosphatases as well as their anchoring and regulatory subunits that fine-tune Ca-handling in specific SR sub-compartments. SR Ca-transport is mediated by the SR Ca-ATPase (SERCA2a) and its regulatory phosphoprotein, phospholamban (PLN). Dephosphorylated PLN is an inhibitor of SERCA2a and phosphorylation by protein kinase A (PKA) or calcium-calmodulin-dependent protein kinases (CAMKII) relieves these inhibitory effects. Recent studies identified additional regulatory proteins, associated with PLN, that control SR Ca-transport. These include the inhibitor-1 (I-1) of protein phosphatase 1 (PP1), the small heat shock protein 20 (Hsp20) and the HS-1 associated protein X-1 (HAX1). In addition, the intra-luminal histidine-rich calcium binding protein (HRC) has been shown to interact with both SERCA2a and triadin. Notably, there is physical and direct interaction between these protein players, mediating a fine-cross talk between SR Ca-uptake, storage and release. Importantly, regulation of SR Ca-cycling by the PLN/SERCA interactome does not only impact cardiomyocyte contractility, but also survival and remodeling. Indeed, naturally occurring variants in these Ca-cycling genes modulate their activity and interactions with other protein partners, resulting in depressed contractility and accelerated remodeling. These genetic variants may serve as potential prognostic or diagnostic markers in cardiac pathophysiology.
Keywords: Calcium; Contractility; Heart failure; Human variants; Phospholamban; Sarcoplasmic reticulum.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
References
-
- Steenaart NA, Ganim JR, Di Salvo J, Kranias EG. The phospholamban phosphatase associated with cardiac sarcoplasmic reticulum is a type 1 enzyme. Arch Biochem Biophys. 1992;293(1):17–24. - PubMed
-
- Pathak A, del Monte F, Zhao W, Shultz JE, Lorenz JN, Bodi I, et al. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96(7):756–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

