Significantly enhanced heme retention ability of myoglobin engineered to mimic the third covalent linkage by nonaxial histidine to heme (vinyl) in synechocystis hemoglobin
- PMID: 25451928
- PMCID: PMC4303654
- DOI: 10.1074/jbc.M114.603225
Significantly enhanced heme retention ability of myoglobin engineered to mimic the third covalent linkage by nonaxial histidine to heme (vinyl) in synechocystis hemoglobin
Abstract
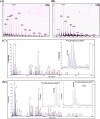
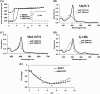
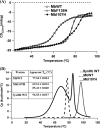
Heme proteins, which reversibly bind oxygen and display a particular fold originally identified in myoglobin (Mb), characterize the "hemoglobin (Hb) superfamily." The long known and widely investigated Hb superfamily, however, has been enriched by the discovery and investigation of new classes and members. Truncated Hbs typify such novel classes and exhibit a distinct two-on-two α-helical fold. The truncated Hb from the freshwater cyanobacterium Synechocystis exhibits hexacoordinate heme chemistry and bears an unusual covalent bond between the nonaxial His(117) and a heme porphyrin 2-vinyl atom, which remains tightly associated with the globin unlike any other. It seems to be the most stable Hb known to date, and His(117) is the dominant force holding the heme. Mutations of amino acid residues in the vicinity did not influence this covalent linkage. Introduction of a nonaxial His into sperm whale Mb at the topologically equivalent position and in close proximity to vinyl group significantly increased the heme stability of this prototype globin. Reversed phase chromatography, electrospray ionization-MS, and MALDI-TOF analyses confirmed the presence of covalent linkage in Mb I107H. The Mb mutant with the engineered covalent linkage was stable to denaturants and exhibited ligand binding and auto-oxidation rates similar to the wild type protein. This indeed is a novel finding and provides a new perspective to the evolution of Hbs. The successful attempt at engineering heme stability holds promise for the production of stable Hb-based blood substitute.
Keywords: Engineered Heme Affinity; Engineering Heme Stability in Myoglobin; Heme; Mimic of Third Covalent Linkage to Heme; Mutagenesis; Myoglobin; Myoglobin with Low Heme Dissociation; Protein Engineering; Protein Stability; Synechocystis Hemoglobin.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Falcón L. I., Magallón S., Castillo A. (2010) Dating the cyanobacterial ancestor of the chloroplast. ISME J. 4, 777–783 - PubMed
-
- Olson J. M., Blankenship R. E. (2004) Thinking about the evolution of photosynthesis. Photosynth. Res. 80, 373–386 - PubMed
-
- Hoy J. A., Kundu S., Trent J. T., 3rd, Ramaswamy S., Hargrove M. S. (2004) The crystal structure of Synechocystis hemoglobin with a covalent heme linkage. J. Biol. Chem. 279, 16535–16542 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

