Effects of prefrontal cortex and hippocampal NMDA NR1-subunit deletion on complex cognitive and social behaviors
- PMID: 25452020
- PMCID: PMC4344942
- DOI: 10.1016/j.brainres.2014.10.037
Effects of prefrontal cortex and hippocampal NMDA NR1-subunit deletion on complex cognitive and social behaviors
Abstract
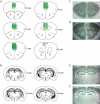
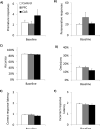
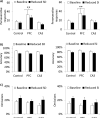
Glutamate N-methyl-D-aspartate receptors (NMDARs) in the medial prefrontal cortex (mPFC) and hippocampus may play an integral role in complex cognitive and social deficits associated with a number of psychiatric illnesses including autism, mood disorders, and schizophrenia. We used localized infusions of adeno-associated virus Cre-recombinase in adult, targeted knock-in mice with loxP sites flanking exons 11-22 of the NR1 gene to investigate the effects of chronic NMDAR dysfunction in the mPFC and CA3 hippocampus on cognitive and social behavior. A 5-choice serial reaction time task (5-CSRTT) was used to monitor aspects of cognitive function that included attention and response inhibition. Social behavior was assessed using Crowley׳s sociability and preference for social novelty protocol. Chronic NMDAR dysfunction localized to the anterior cingulate/prelimbic mPFC or dorsal CA3 hippocampus differentially affected the response inhibition and social interaction. mPFC NR1-deletion increased perseverative responding in the 5-CSRTT and enhanced preference for social novelty, whereas CA3 NR1-deletion increased premature responding in the 5-CSRTT and decreased social approach behavior. These findings suggest that mPFC and CA3 NMDARs play selective roles in regulating compulsive and impulsive behavior, respectively. Furthermore, these findings are consistent with emerging evidence that these behaviors are mediated by distinct, albeit overlapping, neural circuits. Our data also suggest that NMDARs in these regions uniquely contribute to the expression of normal social behavior. In this case, mPFC and CA3 NMDARs appear to inhibit and facilitate aspects of social interaction, respectively. The latter dissociation raises the possibility that distinct circuits contribute to the expression of social intrusiveness and impoverished social interaction.
Keywords: Attention; Hippocampus; NMDA receptor; Prefrontal cortex; Response inhibition; Social interaction.
Published by Elsevier B.V.
Figures



Similar articles
-
Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats.Neuropsychopharmacology. 2006 Apr;31(4):757-67. doi: 10.1038/sj.npp.1300893. Neuropsychopharmacology. 2006. PMID: 16192987
-
Downregulation of 5-hydroxytryptamine7 receptor in the medial prefrontal cortex ameliorates impulsive actions in animal models of schizophrenia.Behav Brain Res. 2018 Apr 2;341:212-223. doi: 10.1016/j.bbr.2017.12.023. Epub 2017 Dec 24. Behav Brain Res. 2018. PMID: 29278697
-
Prefrontal NMDA receptors expressed in excitatory neurons control fear discrimination and fear extinction.Neurobiol Learn Mem. 2015 Mar;119:52-62. doi: 10.1016/j.nlm.2014.12.012. Epub 2015 Jan 20. Neurobiol Learn Mem. 2015. PMID: 25615540 Free PMC article.
-
Prefrontal cortical circuits in social behaviors: an overview.J Zhejiang Univ Sci B. 2024 Nov 15;25(11):941-955. doi: 10.1631/jzus.B2300743. J Zhejiang Univ Sci B. 2024. PMID: 39626878 Free PMC article. Review.
-
The role of the hippocampo-prefrontal cortex system in phencyclidine-induced psychosis: a model for schizophrenia.J Physiol Paris. 2013 Dec;107(6):434-40. doi: 10.1016/j.jphysparis.2013.06.002. Epub 2013 Jun 17. J Physiol Paris. 2013. PMID: 23792022 Review.
Cited by
-
Hippocampal-prefrontal circuit and disrupted functional connectivity in psychiatric and neurodegenerative disorders.Biomed Res Int. 2015;2015:810548. doi: 10.1155/2015/810548. Epub 2015 Apr 1. Biomed Res Int. 2015. PMID: 25918722 Free PMC article. Review.
-
Neuroprotection of Radiosensitive Juvenile Mice by Ultra-High Dose Rate FLASH Irradiation.Cancers (Basel). 2020 Jun 24;12(6):1671. doi: 10.3390/cancers12061671. Cancers (Basel). 2020. PMID: 32599789 Free PMC article.
-
Aberrant glutamatergic systems underlying impulsive behaviors: Insights from clinical and preclinical research.Prog Neuropsychopharmacol Biol Psychiatry. 2024 Dec 20;135:111107. doi: 10.1016/j.pnpbp.2024.111107. Epub 2024 Aug 2. Prog Neuropsychopharmacol Biol Psychiatry. 2024. PMID: 39098647 Review.
-
Frequency-specific medial septal nucleus deep brain stimulation improves spatial memory in MK-801-treated male rats.Neurobiol Dis. 2022 Aug;170:105756. doi: 10.1016/j.nbd.2022.105756. Epub 2022 May 16. Neurobiol Dis. 2022. PMID: 35584727 Free PMC article.
-
Dlgap1 knockout mice exhibit alterations of the postsynaptic density and selective reductions in sociability.Sci Rep. 2018 Feb 2;8(1):2281. doi: 10.1038/s41598-018-20610-y. Sci Rep. 2018. PMID: 29396406 Free PMC article.
References
-
- Abela AR, Dougherty SD, Fagen ED, Hill CJ, Chudasama Y. Inhibitory control deficits in rats with ventral hippocampal lesions. Cereb Cortex. 2013;23:1396–409. - PubMed
-
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology (Berl) 2007 - PubMed
-
- Avale ME, Chabout J, Pons S, Serreau P, De Chaumont F, Olivo-Marin JC, Bourgeois JP, Maskos U, Changeux JP, Granon S. Prefrontal nicotinic receptors control novel social interaction between mice. FASEB J. 2011;25:2145–55. - PubMed
-
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. - PubMed
-
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

