Ataxia is the major neuropathological finding in arylsulfatase G-deficient mice: similarities and dissimilarities to Sanfilippo disease (mucopolysaccharidosis type III)
- PMID: 25452429
- PMCID: PMC4355020
- DOI: 10.1093/hmg/ddu603
Ataxia is the major neuropathological finding in arylsulfatase G-deficient mice: similarities and dissimilarities to Sanfilippo disease (mucopolysaccharidosis type III)
Abstract
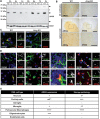
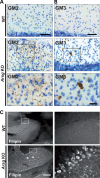
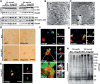
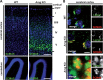
Deficiency of arylsulfatase G (ARSG) leads to a lysosomal storage disease in mice resembling biochemical and pathological features of the mucopolysaccharidoses and particularly features of mucopolysaccharidosis type III (Sanfilippo syndrome). Here we show that Arsg KO mice share common neuropathological findings with other Sanfilippo syndrome models and patients, but they can be clearly distinguished by the limitation of most phenotypic alterations to the cerebellum, presenting with ataxia as the major neurological finding. We determined in detail the expression of ARSG in the central nervous system and observed highest expression in perivascular macrophages (which are characterized by abundant vacuolization in Arsg KO mice) and oligodendrocytes. To gain insight into possible mechanisms leading to ataxia, the pathology in older adult mice (>12 months) was investigated in detail. This study revealed massive loss of Purkinje cells and gliosis in the cerebellum, and secondary accumulation of glycolipids like GM2 and GM3 gangliosides and unesterified cholesterol in surviving Purkinje cells, as well as neurons of some other brain regions. The abundant presence of ubiquitin and p62-positive aggregates in degenerating Purkinje cells coupled with the absence of significant defects in macroautophagy is consistent with lysosomal membrane permeabilization playing a role in the pathogenesis of Arsg-deficient mice and presumably Sanfilippo disease in general. Our data delineating the phenotype of mucopolysaccharidosis IIIE in a mouse KO model should help in the identification of possible human cases of this disease.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Valstar M.J., Ruijter G.J., van Diggelen O.P., Poorthuis B.J., Wijburg F.A. Sanfilippo syndrome: a mini-review. J. Inherit. Metab. Dis. 2008;31:240–252. - PubMed
-
- Neufeld E.F., Muenzer J. In: The Metabolic and Molecular Bases of Inherited Diseases. Scriver C.R., editor. New York, NY: McGraw-Hill; 2001. pp. 3421–3452. The Mucopolysaccharidoses.
-
- Muenzer J. Mucopolysaccharidoses. Adv. Pediatr. 1986;33:269–302. - PubMed
-
- Martin J.J., Ceuterick C., Van Dessel G., Lagrou A., Dierick W. Two cases of mucopolysaccharidosis type III (Sanfilippo). An anatomopathological study. Acta Neuropathol. 1979;46:185–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

