A defect in early myogenesis causes Otitis media in two mouse models of 22q11.2 Deletion Syndrome
- PMID: 25452432
- PMCID: PMC4355021
- DOI: 10.1093/hmg/ddu604
A defect in early myogenesis causes Otitis media in two mouse models of 22q11.2 Deletion Syndrome
Abstract
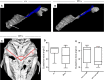
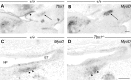
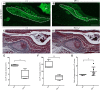
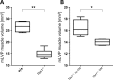
Otitis media (OM), the inflammation of the middle ear, is the most common disease and cause for surgery in infants worldwide. Chronic Otitis media with effusion (OME) often leads to conductive hearing loss and is a common feature of a number of craniofacial syndromes, such as 22q11.2 Deletion Syndrome (22q11.2DS). OM is more common in children because the more horizontal position of the Eustachian tube (ET) in infants limits or delays clearance of middle ear effusions. Some mouse models with OM have shown alterations in the morphology and angle of the ET. Here, we present a novel mechanism in which OM is caused not by a defect in the ET itself but in the muscles that control its function. Our results show that in two mouse models of 22q11.2DS (Df1/+ and Tbx1(+/-)) presenting with bi- or unilateral OME, the fourth pharyngeal arch-derived levator veli palatini muscles were hypoplastic, which was associated with an earlier altered pattern of MyoD expression. Importantly, in mice with unilateral OME, the side with the inflammation was associated with significantly smaller muscles than the contralateral unaffected ear. Functional tests examining ET patency confirmed a reduced clearing ability in the heterozygous mice. Our findings are also of clinical relevance as targeting hypoplastic muscles might present a novel preventative measure for reducing the high rates of OM in 22q11.2DS patients.
© The Author 2014. Published by Oxford University Press.
Figures




References
-
- Thomas J.A., Graham J.M. Chromosomes 22q1l deletion syndrome: an update and review for the primary pediatrician. Clin. Pediatr. (Phila) 1997;36:253–266. - PubMed
-
- Ryan A.K., Goodship J.A., Wilson D.I., Philip N., Levy A., Seidel H., Schuffenhauer S., Oechsler H., Belohradsky B., Prieur M., et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J. Med. Genet. 1997;34:798–804. - PMC - PubMed
-
- Puech A., Saint-Jore B., Funke B., Gilbert D.J., Sirotkin H., Copeland N.G., Jenkins N.A., Kucherlapati R., Morrow B., Skoultchi A.I. Comparative mapping of the human 22q11 chromosomal region and the orthologous region in mice reveals complex changes in gene organization. Proc. Natl Acad. Sci. USA. 1997;94:14608–14613. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

