Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors
- PMID: 25452437
- PMCID: PMC4372849
- DOI: 10.1200/JCO.2014.57.1547
Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors
Abstract
Purpose: Arthralgia occurs in up to 50% of breast cancer survivors treated with aromatase inhibitors (AIs) and is the most common reason for poor AI adherence. We conducted, in 121 breast cancer survivors receiving an AI and reporting arthralgia, a yearlong randomized trial of the impact of exercise versus usual care on arthralgia severity.
Patients and methods: Eligibility criteria included receiving an AI for at least 6 months, reporting ≥ 3 of 10 for worst joint pain on the Brief Pain Inventory (BPI), and reporting < 90 minutes per week of aerobic exercise and no strength training. Participants were randomly assigned to exercise (150 minutes per week of aerobic exercise and supervised strength training twice per week) or usual care. The BPI, Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index, and Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire were completed at baseline and at 3, 6, 9, and 12 months. Intervention effects were evaluated using mixed-model repeated measures analysis, with change at 12 months as the primary end point.
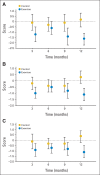
Results: Over 12 months, women randomly assigned to exercise (n = 61) attended 70% (± standard deviation [SD], 28%) of resistance training sessions and increased their exercise by 159 (± SD, 136) minutes per week. Worst joint pain scores decreased by 1.6 points (29%) at 12 months among women randomly assigned to exercise versus a 0.2-point increase (3%) among those receiving usual care (n = 60; P < .001). Pain severity and interference, as well as DASH and WOMAC pain scores, also decreased significantly at 12 months in women randomly assigned to exercise, compared with increases for those receiving usual care (all P < .001).
Conclusion: Exercise led to improvement in AI-induced arthralgia in previously inactive breast cancer survivors.
Trial registration: ClinicalTrials.gov NCT02056067.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
References
-
- Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after 5 years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. - PubMed
-
- Clarke M. Meta-analyses of adjuvant therapies for women with early breast cancer: The Early Breast Cancer Trialists' Collaborative Group overview. Ann Oncol. 2006;17(suppl 10):x59–x62. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical