Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial
- PMID: 25452452
- PMCID: PMC4806782
- DOI: 10.1200/JCO.2014.59.0703
Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial
Abstract
Purpose: Nivolumab is a fully human immunoglobulin G4 programmed death-1 immune checkpoint inhibitor antibody that restores T-cell immune activity. This phase II trial assessed the antitumor activity, dose-response relationship, and safety of nivolumab in patients with metastatic renal cell carcinoma (mRCC).
Patients and methods: Patients with clear-cell mRCC previously treated with agents targeting the vascular endothelial growth factor pathway were randomly assigned (blinded ratio of 1:1:1) to nivolumab 0.3, 2, or 10 mg/kg intravenously once every 3 weeks. The primary objective was to evaluate the dose-response relationship as measured by progression-free survival (PFS); secondary end points included objective response rate (ORR), overall survival (OS), and safety.
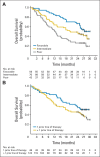
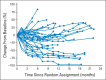
Results: A total of 168 patients were randomly assigned to the nivolumab 0.3- (n = 60), 2- (n = 54), and 10-mg/kg (n = 54) cohorts. One hundred eighteen patients (70%) had received more than one prior systemic regimen. Median PFS was 2.7, 4.0, and 4.2 months, respectively (P = .9). Respective ORRs were 20%, 22%, and 20%. Median OS was 18.2 months (80% CI, 16.2 to 24.0 months), 25.5 months (80% CI, 19.8 to 28.8 months), and 24.7 months (80% CI, 15.3 to 26.0 months), respectively. The most common treatment-related adverse event (AE) was fatigue (24%, 22%, and 35%, respectively). Nineteen patients (11%) experienced grade 3 to 4 treatment-related AEs.
Conclusion: Nivolumab demonstrated antitumor activity with a manageable safety profile across the three doses studied in mRCC. No dose-response relationship was detected as measured by PFS. These efficacy and safety results in mRCC support study in the phase III setting.
Trial registration: ClinicalTrials.gov NCT01354431.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures



References
-
- Escudier B, Eisen T, Porta C, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii65–vii71. - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Kidney cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. - PubMed
-
- Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

