Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures
- PMID: 25452496
- PMCID: PMC4339118
- DOI: 10.15252/embj.201489652
Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures
Abstract
Transmembrane guanylyl cyclases (GCs), with activity regulated by peptide ligands and/or calcium-binding proteins, are essential for various physiological and sensory processes. The mode of activation of the GC subtype GC-G, which is expressed in neurons of the Grueneberg ganglion that respond to cool temperatures, has been elusive. In searching for appropriate stimuli to activate GC-G, we found that its enzymatic activity is directly stimulated by cool temperatures. In this context, it was observed that dimerization/oligomerization of GC-G, a process generally considered as critical for enzymatic activity of GCs, is strongly enhanced by coolness. Moreover, heterologous expression of GC-G in cultured cells rendered these cells responsive to coolness; thus, the protein might be a sensor for cool temperatures. This concept is supported by the observation of substantially reduced coolness-induced response of Grueneberg ganglion neurons and coolness-evoked ultrasonic vocalization in GC-G-deficient mouse pups. GC-G may be a novel thermosensory protein with functional implications for the Grueneberg ganglion, a sensory organ responding to cool temperatures.
Keywords: Grueneberg ganglion; chemosensory; cyclic guanosine monophosphate; transmembrane guanylyl cyclase GC‐G; ultrasound vocalization.
© 2014 Academia Sinica.
Figures
Coolness stimulates intracellular cyclic guanosine monophosphate (cGMP) accumulation in HEK-293T cells expressing GC-G. Two days after transfection of empty vector or GC-G-encoding plasmid, cells were exposed to ambient temperatures (37 or 15°C) for 20 min and cellular cGMP concentration was measured.
Temperature dependence of GC-G activity. Membrane preparations from HEK-293T cells transfected with empty vector or GC-G-encoding plasmid were used to determine cyclase activity at the indicated temperatures for 20 min (asterisks denote values significantly elevated compared to 37°C).
GC-G is the only murine transmembrane GC that can be stimulated by cool temperatures. Expression plasmids encoding FLAG-tagged GC subtypes GC-A to GC-G were transfected into HEK-293T cells. Two days after transfection, membrane protein fractions were prepared and used for cyclase activity assays at 37 or 15°C. Protein expression of each receptor GC was confirmed by Western blot (WB) analysis with anti-FLAG antibody.
The H+CYC domain is critical for stimulation by coolness. The domain structure of full-length (FL) GC-G and its mutant variants is shown in the upper panel. A FLAG tag was added at the N-terminus of each protein. The ΔECD+KLD mutant protein lacks amino acids 73–455 and 556–833; the ΔCYC variant lacks amino acids 834–1,003. The H+CYC mutant protein contains amino acids 806–1,100. ECD, extracellular domain; KLD, kinase-like domain; SP, signal peptide; TM, transmembrane region. HEK-293T cells expressing the indicated GC-G constructs were incubated at 37 or 15°C for 20 min, then intracellular cGMP concentration was measured (lower panel).


A Representation of the domain structure from different C-terminal fragments of GC-G analyzed for coolness-induced activity (amino acids of GC-G encoded in each expression plasmid are indicated).
B, C Protein expression and coolness-stimulated cGMP synthesis of the above shown divergent C-terminal fragments of GC-G with intact or ablated hinge (H) region. The expression plasmids encoding the H+CYC domain or a deletion construct (Δ1–Δ2) tagged with a FLAG epitope were transfected into HEK-293T cells. Two days after transfection, cells were exposed to the ambient temperatures (37 or 15°C) for 20 min and cellular cGMP concentration was measured (C). Protein expression of each construct was confirmed by Western blot (WB) analysis with anti-FLAG antibody (B).
D A recombinant protein containing the H+CYC domain of GC-G acquires coolness-evoked activity. Diagram of recombinant GST.GC-G-CYC protein containing the H+CYC domain of GC-G (amino acids 849–1,078) compared to the domain structure of full-length GC-G (upper panel). The purified GST.GC-G-CYC protein was exposed to the indicated temperatures for 20 min, and GST.GC-G-CYC cyclase activity was measured (lower panel; asterisks denote values significantly elevated compared to 37°C).
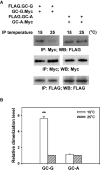
HEK-293T cells were co-transfected with plasmids encoding two differential epitope-tagged (FLAG or Myc) versions of GC-G or GC-A, respectively. After 2 days, transfected cells were lysed with detergent and lysates were subjected to immunoprecipitation (IP) for 2 h with anti-Myc antibody at two different temperatures (15 or 25°C), followed by Western blotting (WB) using anti-FLAG antibody to determine the amount of dimerized/oligomerized GC proteins (upper panel). These approaches demonstrated that in contrast to GC-A, homomeric protein/protein association of GC-G seems to be enhanced by cool temperatures (upper panel). Cell lysates werealso precipitated and immunoblotted with the anti-Myc antibody, demonstrating that cooling does not interfere with immunoprecipitation of Myc-tagged GC-G via the anti-Myc antibody (middle panel). Likewise, precipitation and immunoblotting with the anti-FLAG antibody yielded bands of similar intensity for 15 and 25°C, respectively (lower panel).
Relative quantification of homomeric protein/protein association of GC-G and GC-A was performed by densitometric scanning. Each intensity value from the upper panel was subsequently normalized to the total protein levels as determined in the middle and the lower panel of Fig4A (intensity of the immunoreactive bands obtained with the anti-Myc or anti-FLAG antibody, respectively). Relative dimerization level at 15°C was further calculated by normalizing to values obtained at 25°C; the latter were set as 1. Results are means ± SD of three experiments, **P < 0.01 (compared to 25°C).

A-H In situ hybridization experiments with an antisense probe for c-Fos on coronal sections through the GG of wild-type (WT; left panel) or GC-G-KO (right panel) mouse pups (P0–P4) exposed to warm (35°C; A–D) or cool (22°C; E–H) ambient temperature for 2 h. Panels (C, D, G, H) show higher magnifications of the boxed areas in (A, B, E, F). At 35°C (A–D), no c-Fos expression was detectable in the GG of both WT (A, C) and GC-G-KO (B, D) individuals. Upon exposure to 22°C, strong c-Fos expression was observed in the GG of WT pups (E, G), whereas c-Fos expression was barely detectable in the GG of GC-G-KO animals (F, H). The data shown are representative of 10 independent experiments. Pups originated from six different litters for each genotype. Scale bars: 200 μm in (A, B, E, F); 50 μm in (C, D, G, H).
I Quantification of c-Fos-positive GG cells in WT and GC-G-KO pups on exposure to 22°C for 2 h. All stained cells on every section along the rostrocaudal extent of the GG were counted; the results shown are based on the above-mentioned 10 experiments. The number of c-Fos-positive cells in the GG of a GC-G-KO mouse was determined relative to that in a concomitantly processed WT pup; the latter number was set to 100%. Data are mean ± SD (n = 11 in each genotype), **P < 0.0001.
J-N Calcium imaging of coronal sections through the GG of olfactory marker protein-green fluorescent protein (OMP-GFP)/GC-G+/+ or OMP-GFP/GC-G-KO pups (P1–P4). High magnification image (J) of a tissue slice through the GG of an OMP-GFP pup with GG neurons labeled by intrinsic GFP fluorescence (GFP fluorescence was merged with the transmitted-light channel). Cells analyzed are circled in blue (GFP-negative) or red (GFP-positive). NC, nasal cavity. Scale bar: 30 μm. (K–M) Representative ratiometric Ca2+ transients induced by cooling from 37 to 15°C in GFP-negative non-neuronal cells (K) and in GFP-positive GG neurons from OMP-GFP/GC-G+/+ (L) and OMP-GFP/GC-G-KO pups (M). The numbers in the bottom right hand corners are the number of cells with Ca2+ transient similar to what is shown in the respective graph (left) and total number of measured cells (right). (N) Quantification of coolness-induced ΔF in GG neurons from OMP-GFP/GC-G+/+ and OMP-GFP/GC-G-KO mice. Coolness-induced ΔF was calculated by subtracting the baseline fluorescence ratio (340/380 nm) at 37°C from the peak fluorescence ratio (340/380 nm) measured at 15°C. For OMP-GFP/GC-G+/+, the 29 coolness-responsive neurons (black bar) of all 54 analyzed neurons (gray bar) from five slices (obtained from different animals) were analyzed. For OMP-GFP/GC-G-KO, the 50 analyzed neurons (shaded bar) from five slices (obtained from different animals) were analyzed. Data are mean ± SD, **P < 0.01.

A Reduced responsive rate of coolness-stimulated USV in GC-G-KO pups. Pups were removed from their littermates and dams at postnatal (P) day 2, 4, and 6 and exposed to 15°C. USV was measured by use of the Anabat SD1 Bat detector. The number of responsive pups versus total number of pups tested for each WT and GC-G-KO group at different ages is indicated on the top of each bar. *P < 0.05.
B, C Total number of USV calls (B) and response latency (C) in GC-G-KO and WT pups at P2. The horizontal bars indicate the mean. *P < 0.05; **P < 0.01.
Comment in
-
It's cold, mom! It's cyclic GMP.EMBO J. 2015 Feb 3;34(3):270-2. doi: 10.15252/embj.201490639. Epub 2015 Jan 2. EMBO J. 2015. PMID: 25555793 Free PMC article.
References
-
- Allin JT, Banks EM. Effects of temperature on ultrasound production by infant albino rats. Dev Psychobiol. 1971;4:149–156. - PubMed
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. - PubMed
-
- Blumberg MS, Efimova IV, Alberts JR. Ultrasonic vocalizations by rat pups: the primary importance of ambient temperature and the thermal significance of contact comfort. Dev Psychobiol. 1992;25:229–250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

