Life-long in vivo cell-lineage tracing shows that no oogenesis originates from putative germline stem cells in adult mice
- PMID: 25453063
- PMCID: PMC4273382
- DOI: 10.1073/pnas.1421047111
Life-long in vivo cell-lineage tracing shows that no oogenesis originates from putative germline stem cells in adult mice
Abstract
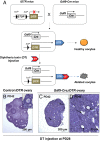
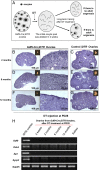
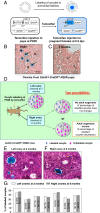
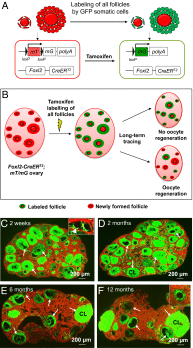
Whether or not oocyte regeneration occurs in adult life has been the subject of much debate. In this study, we have traced germ-cell lineages over the life spans of three genetically modified mouse models and provide direct evidence that oogenesis does not originate from any germline stem cells (GSCs) in adult mice. By selective ablation of all existing oocytes in a Gdf9-Cre;iDTR mouse model, we have demonstrated that no new germ cells were ever regenerated under pathological conditions. By in vivo tracing of oocytes and follicles in the Sohlh1-CreER(T2);R26R and Foxl2-CreER(T2);mT/mG mouse models, respectively, we have shown that the initial pool of oocytes is the only source of germ cells throughout the life span of the mice and that no adult oogenesis ever occurs under physiological conditions. Our findings clearly show that there are no GSCs that contribute to adult oogenesis in mice and that the initial pool of oocytes formed in early life is the only source of germ cells throughout the entire reproductive life span.
Keywords: follicle tracing; genetically modified mouse models; life-long observations; no postnatal oocyte regeneration; oocyte tracing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Seeing is believing: no adult oogenesis in mammals.Cell Cycle. 2015;14(7):935-6. doi: 10.1080/15384101.2015.1010974. Cell Cycle. 2015. PMID: 25714625 Free PMC article. No abstract available.
References
-
- Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;6:63–109.
-
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. - PubMed
-
- Zou K, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11(5):631–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

