Neuromolecular responses to social challenge: common mechanisms across mouse, stickleback fish, and honey bee
- PMID: 25453090
- PMCID: PMC4273386
- DOI: 10.1073/pnas.1420369111
Neuromolecular responses to social challenge: common mechanisms across mouse, stickleback fish, and honey bee
Abstract
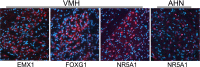
Certain complex phenotypes appear repeatedly across diverse species due to processes of evolutionary conservation and convergence. In some contexts like developmental body patterning, there is increased appreciation that common molecular mechanisms underlie common phenotypes; these molecular mechanisms include highly conserved genes and networks that may be modified by lineage-specific mutations. However, the existence of deeply conserved mechanisms for social behaviors has not yet been demonstrated. We used a comparative genomics approach to determine whether shared neuromolecular mechanisms could underlie behavioral response to territory intrusion across species spanning a broad phylogenetic range: house mouse (Mus musculus), stickleback fish (Gasterosteus aculeatus), and honey bee (Apis mellifera). Territory intrusion modulated similar brain functional processes in each species, including those associated with hormone-mediated signal transduction and neurodevelopment. Changes in chromosome organization and energy metabolism appear to be core, conserved processes involved in the response to territory intrusion. We also found that several homologous transcription factors that are typically associated with neural development were modulated across all three species, suggesting that shared neuronal effects may involve transcriptional cascades of evolutionarily conserved genes. Furthermore, immunohistochemical analyses of a subset of these transcription factors in mouse again implicated modulation of energy metabolism in the behavioral response. These results provide support for conserved genetic "toolkits" that are used in independent evolutions of the response to social challenge in diverse taxa.
Keywords: NF-κB signaling; aggression; brain metabolism; genetic hotspot.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Stern DL. The genetic causes of convergent evolution. Nat Rev Genet. 2013;14(11):751–764. - PubMed
-
- Martin A, Orgogozo V. The loci of repeated evolution: A catalog of genetic hotspots of phenotypic variation. Evolution. 2013;67(5):1235–1250. - PubMed
-
- Gellon G, McGinnis W. Shaping animal body plans in development and evolution by modulation of Hox expression patterns. BioEssays. 1998;20(2):116–125. - PubMed
-
- Toth AL, Robinson GE. Evo-devo and the evolution of social behavior. Trends Genet. 2007;23(7):334–341. - PubMed
-
- Sumner S. The importance of genomic novelty in social evolution. Mol Ecol. 2014;23(1):26–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

