Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial
- PMID: 25453457
- PMCID: PMC4284947
- DOI: 10.1016/S1474-4422(14)70224-8
Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial
Abstract
Background: The primary outcome results for the SPS3 trial suggested that a lower systolic target blood pressure (<130 mm Hg) might be beneficial for reducing the risk of recurrent stroke compared with a higher target (130-149 mm Hg), but that the addition of clopidogrel to aspirin was not beneficial compared with aspirin plus placebo. In this prespecified secondary outcome analysis of the SPS3 trial, we aimed to assess whether blood pressure reduction and dual antiplatelet treatment affect changes in cognitive function over time in patients with cerebral small vessel disease.
Methods: In the SPS3 trial, patients with recent (within 6 months) symptomatic lacunar infarcts from 81 centres in North America, Latin America, and Spain were randomly assigned, in a two-by-two factorial design, to target levels of systolic blood pressure (1:1; 130-149 mm Hg vs <130 mm Hg; open-label) and to a once-daily antiplatelet treatment (1:1; aspirin 325 mg plus clopidogrel 75 mg vs aspirin 325 mg plus placebo; double-blind). For this analysis, the main cognitive outcome was change in Cognitive Abilities Screening Instrument (CASI) during follow-up. Patients were tested annually for up to 5 years, during which time the mean difference in systolic blood pressure was 11 mm Hg (SD 16) between the two targets (138 mm Hg vs 127 mm Hg at 1 year). We used linear mixed models to compare changes in CASI Z scores over time. The SPS3 trial is registered with ClinicalTrials.gov, number NCT00059306.
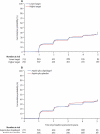
Findings: The study took place between March 23, 2003, and April 30, 2012. 2916 of 3020 SPS3 participants (mean age 63 years [SD 11]) with CASI scores at study entry were included in the analysis, with a median follow-up of 3·0 years (IQR 1·0-4·9). Mean changes in CASI Z scores from study entry to assessment at years 1 (n=2472), 2 (n=1968), 3 (n=1521), 4 (n=1135), and 5 (n=803) were 0·12 (SD 0·83), 0·15 (0·84), 0·16 (0·95), 0·19 (0·99), and 0·14 (1·09), respectively. Changes in CASI Z scores over time did not differ between assigned antiplatelet groups (p=0·858) or between assigned blood pressure target groups (p=0·520). There was no interaction between assigned antiplatelet groups and assigned blood pressure target groups and change over time (p=0·196).
Interpretation: Cognitive function is not affected by short-term dual antiplatelet treatment or blood pressure reduction in fairly young patients with recent lacunar stroke. Future studies of cognitive function after stroke should be of longer duration or focus on patients with higher rates of cognitive decline.
Funding: US National Institute of Neurological Disorders and Stroke.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Blood pressure control after stroke: too little, too late, or too soon to tell?Lancet Neurol. 2014 Dec;13(12):1162-3. doi: 10.1016/S1474-4422(14)70180-2. Epub 2014 Oct 23. Lancet Neurol. 2014. PMID: 25453448 No abstract available.
References
-
- Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4(11):752–9. - PubMed
-
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–18. - PubMed
-
- Skoog I, Gustafson D. Update on hypertension and Alzheimer's disease. Neurol Res. 2006;28(6):605–11. - PubMed
-
- Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

