Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds
- PMID: 25453950
- PMCID: PMC4312192
- DOI: 10.1016/j.biomaterials.2014.10.017
Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds
Abstract
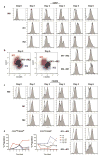
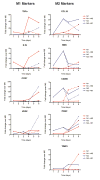
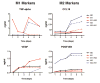
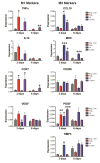
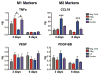
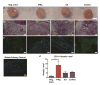
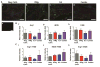
In normal tissue repair, macrophages exhibit a pro-inflammatory phenotype (M1) at early stages and a pro-healing phenotype (M2) at later stages. We have previously shown that M1 macrophages initiate angiogenesis while M2 macrophages promote vessel maturation. Therefore, we reasoned that scaffolds that promote sequential M1 and M2 polarization of infiltrating macrophages should result in enhanced angiogenesis and healing. To this end, we first analyzed the in vitro kinetics of macrophage phenotype switch using flow cytometry, gene expression, and cytokine secretion analysis. Then, we designed scaffolds for bone regeneration based on modifications of decellularized bone for a short release of interferon-gamma (IFNg) to promote the M1 phenotype, followed by a more sustained release of interleukin-4 (IL4) to promote the M2 phenotype. To achieve this sequential release profile, IFNg was physically adsorbed onto the scaffolds, while IL4 was attached via biotin-streptavidin binding. Interestingly, despite the strong interactions between biotin and streptavidin, release studies showed that biotinylated IL4 was released over 6 days. These scaffolds promoted sequential M1 and M2 polarization of primary human macrophages as measured by gene expression of ten M1 and M2 markers and secretion of four cytokines, although the overlapping phases of IFNg and IL4 release tempered polarization to some extent. Murine subcutaneous implantation model showed increased vascularization in scaffolds releasing IFNg compared to controls. This study demonstrates that scaffolds for tissue engineering can be designed to harness the angiogenic behavior of host macrophages towards scaffold vascularization.
Keywords: Bone; Cytokines; Immunomodulation; Macrophages; Regenerative medicine; Vascularization.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
-
- Sikavitsas VI, Bancroft GN, Lemoine JJ, Liebschner MA, Dauner M, Mikos AG. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng. 2005;33(1):63–70. - PubMed
-
- Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

