Effect of decision support on missed opportunities for human papillomavirus vaccination
- PMID: 25455116
- PMCID: PMC4254426
- DOI: 10.1016/j.amepre.2014.08.010
Effect of decision support on missed opportunities for human papillomavirus vaccination
Abstract
Background: Missed opportunities for human papilloma virus (HPV) vaccination are common, presenting a barrier to achieving widespread vaccine coverage and preventing infection.
Purpose: To compare the impact of clinician- versus family-focused decision support, none, or both on captured opportunities for HPV vaccination.
Design: Twelve-month cluster randomized controlled trial conducted in 2010-2011.
Setting/participants: Adolescent girls aged 11-17 years due for HPV Dose 1, 2, or 3 receiving care at primary care practices.
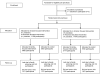
Intervention: Twenty-two primary care practices were cluster randomized to receive a three-part clinician-focused intervention (educational sessions, electronic health record-based alerts, and performance feedback) or none. Within each practice, girls were randomized at the patient level to receive family-focused, automated, educational phone calls or none. Randomization resulted in four groups: clinician-focused, family-focused, combined, or no intervention.
Main outcome measures: Standardized proportions of captured opportunities (due vaccine received at clinician visit) were calculated among girls in each study arm. Analyses were conducted in 2013.
Results: Among 17,016 adolescent girls and their 32,472 visits (14,247 preventive, 18,225 acute), more HPV opportunities were captured at preventive than acute visits (36% vs 4%, p<0.001). At preventive visits, the clinician intervention increased captured opportunities by 9 percentage points for HPV-1 and 6 percentage points for HPV-3 (p≤0.01), but not HPV-2. At acute visits, the clinician and combined interventions significantly improved captured opportunities for all three doses (p≤0.01). The family intervention was similar to none. Results differed by practice setting; at preventive visits, the clinician intervention was more effective for HPV-1 in suburban than urban settings, whereas at acute visits, the clinician intervention was more effective for all doses at urban practices.
Conclusions: Clinician-focused decision support is a more effective strategy than family-focused to prevent missed HPV vaccination opportunities. Given the persistence of missed opportunities even in intervention groups, complementary strategies are needed. This study is registered at clinicaltrials.gov NCT01159093.
Copyright © 2014 American Journal of Preventive Medicine. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–93. - PubMed
-
- CDC. Human papillomavirus-associated cancers - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2012;61:258–61. - PubMed
-
- Atkinson W, Wolfe S, Hamborsky J, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th. Washington, D.C.: Public Health Foundation; 2012. Centers for Disease Control and Prevention.
-
- Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. - PubMed
-
- Dunne EF, Markowitz LE, Chesson H, Saraiya M, Gee J, Unger ER. Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

