TCR and CD28 activate the transcription factor NF-κB in T-cells via distinct adaptor signaling complexes
- PMID: 25455592
- PMCID: PMC4286576
- DOI: 10.1016/j.imlet.2014.10.020
TCR and CD28 activate the transcription factor NF-κB in T-cells via distinct adaptor signaling complexes
Abstract
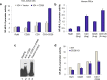
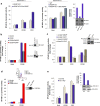
The transcription factor NF-κB is needed for the induction of inflammatory responses in T-cells. Whether its activation by the antigen-receptor and CD28 is mediated by the same or different intracellular signaling pathways has been unclear. Here, using T-cells from various knock-out (Cd28(-/-), adap(-/-)) and knock-in (i.e. Cd28 Y-170F) mice in conjunction with transfected Jurkat T-cells, we show that the TCR and CD28 use distinct pathways to activate NF-κB in T-cells. Anti-CD28 ligation alone activated NF-κB in primary and Jurkat T-cells as measured by NF-κB reporter and EMSA assays. Anti-CD28 also activated NF-κB normally in primary T-cells from adap(-/-) mice, while anti-CD3 stimulation required the adaptor ADAP. Over-expression of ADAP or its binding partner SKAP1 failed to enhance anti-CD28 activation of NF-κB, while ADAP greatly increased anti-CD3 induced NF-κB activity. By contrast, CD28 activation of NF-κB depended on GRB-2 binding to CD28 as seen in CD28 deficient Jurkat T-cells reconstituted with the CD28 YMN-FM mutant, and in primary T-cells from CD28 Y170F mutant knock-in mice. CD28 associated with GRB-2, and GRB-2 siRNA impaired CD28 NF-κB activation. GRB-2 binding partner and guanine nucleotide exchange factor, VAV1, greatly enhanced anti-CD28 driven activation of NF-κB. Further, unlike in the case of anti-CD28, NF-κB activation by anti-CD3 and its cooperation with ADAP was strictly dependent on LAT expression. Overall, we provide evidence that CD28 and the TCR complex regulate NF-κB via different signaling modules of GRB-2/VAV1 and LAT/ADAP pathways respectively.
Keywords: ADAP; Adaptors; GRB-2; NF-κB; VAV-1.
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Rudd C.E. Adaptors and molecular scaffolds in immune cell signaling. Cell. 1999;96:5–8. - PubMed
-
- Samelson L.E. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–394. - PubMed
-
- Weiss A. TCR signal transduction: opening the black box. J Immunol. 2009;183:4821–4827. - PubMed
-
- Dustin M.L., Shaw A.S. Costimulation: building an immunological synapse. Science. 1999;283:649–650. - PubMed
-
- June C.H., Bluestone J.A., Nadler L.M., Thompson C.B. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

