Hormone-induced calcium oscillations depend on cross-coupling with inositol 1,4,5-trisphosphate oscillations
- PMID: 25456123
- PMCID: PMC6469397
- DOI: 10.1016/j.celrep.2014.10.033
Hormone-induced calcium oscillations depend on cross-coupling with inositol 1,4,5-trisphosphate oscillations
Abstract
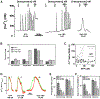
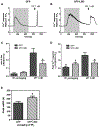
Receptor-mediated oscillations in cytosolic Ca(2+) concentration ([Ca(2+)]i) could originate either directly from an autonomous Ca(2+) feedback oscillator at the inositol 1,4,5-trisphosphate (IP3) receptor or as a secondary consequence of IP3 oscillations driven by Ca(2+) feedback on IP3 metabolism. It is challenging to discriminate these alternatives, because IP3 fluctuations could drive Ca(2+) oscillations or could just be a secondary response to the [Ca(2+)]i spikes. To investigate this problem, we constructed a recombinant IP3 buffer using type-I IP3 receptor ligand-binding domain fused to GFP (GFP-LBD), which buffers IP3 in the physiological range. This IP3 buffer slows hormone-induced [IP3] dynamics without changing steady-state [IP3]. GFP-LBD perturbed [Ca(2+)]i oscillations in a dose-dependent manner: it decreased both the rate of [Ca(2+)]i rise and the speed of Ca(2+) wave propagation and, at high levels, abolished [Ca(2+)]i oscillations completely. These data, together with computational modeling, demonstrate that IP3 dynamics play a fundamental role in generating [Ca(2+)]i oscillations and waves.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


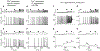
References
-
- Bartlett PJ, Gaspers LD, Pierobon N, and Thomas AP (2014). Calcium-dependent regulation of glucose homeostasis in the liver. Cell Calcium 55, 306–316. - PubMed
-
- Berridge MJ (1993). Inositol trisphosphate and calcium signalling. Nature 361, 315–325. - PubMed
-
- Dupont G, and Goldbeter A (1993). One-pool model for Ca2+ oscillations involving Ca2+ and inositol 1,4,5-trisphosphate as co-agonists for Ca2+ release. Cell Calcium 14, 311–322. - PubMed
-
- Dupont G, Koukoui O, Clair C, Erneux C, Swillens S, and Combettes L (2003). Ca2+ oscillations in hepatocytes do not require the modulation of InsP3 3-kinase activity by Ca2+. FEBS Lett 534, 101–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

