FXR1P limits long-term memory, long-lasting synaptic potentiation, and de novo GluA2 translation
- PMID: 25456134
- PMCID: PMC4254574
- DOI: 10.1016/j.celrep.2014.10.028
FXR1P limits long-term memory, long-lasting synaptic potentiation, and de novo GluA2 translation
Abstract
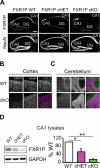
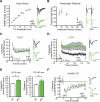
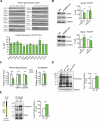
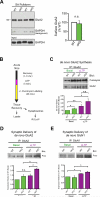
Translational control of mRNAs allows for rapid and selective changes in synaptic protein expression that are required for long-lasting plasticity and memory formation in the brain. Fragile X Related Protein 1 (FXR1P) is an RNA-binding protein that controls mRNA translation in nonneuronal cells and colocalizes with translational machinery in neurons. However, its neuronal mRNA targets and role in the brain are unknown. Here, we demonstrate that removal of FXR1P from the forebrain of postnatal mice selectively enhances long-term storage of spatial memories, hippocampal late-phase long-term potentiation (L-LTP), and de novo GluA2 synthesis. Furthermore, FXR1P binds specifically to the 5' UTR of GluA2 mRNA to repress translation and limit the amount of GluA2 that is incorporated at potentiated synapses. This study uncovers a mechanism for regulating long-lasting synaptic plasticity and spatial memory formation and reveals an unexpected divergent role of FXR1P among Fragile X proteins in brain plasticity.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Bontekoe CJM, McIlwain KL, Nieuwenhuizen IM, Yuva-Paylor L. a, Nellis A, Willemsen R, Fang Z, Kirkpatrick L, Bakker CE, McAninch R, et al. Knockout mouse model for Fxr2: a model for mental retardation. Hum. Mol. Genet. 2002;11:487–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

