The TLQP-21 peptide activates the G-protein-coupled receptor C3aR1 via a folding-upon-binding mechanism
- PMID: 25456411
- PMCID: PMC4353613
- DOI: 10.1016/j.str.2014.10.001
The TLQP-21 peptide activates the G-protein-coupled receptor C3aR1 via a folding-upon-binding mechanism
Abstract
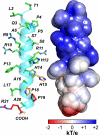
TLQP-21, a VGF-encoded peptide is emerging as a novel target for obesity-associated disorders. TLQP-21 is found in the sympathetic nerve terminals in the adipose tissue and targets the G-protein-coupled receptor complement-3a receptor1 (C3aR1). The mechanisms of TLQP-21-induced receptor activation remain unexplored. Here, we report that TLQP-21 is intrinsically disordered and undergoes a disorder-to-order transition, adopting an α-helical conformation upon targeting cells expressing the C3aR1. We determined that the hot spots for TLQP-21 are located at the C terminus, with mutations in the last four amino acids progressively reducing the bioactivity and, a single site mutation (R21A) or C-terminal amidation abolishing its function completely. Additionally, the human TLQP-21 sequence carrying a S20A substitution activates the human C3aR1 receptor with lower potency compared to the rodent sequence. These studies reveal the mechanism of action of TLQP-21 and provide molecular templates for designing agonists and antagonists to modulate C3aR1 functions.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
The molecular identity of the TLQP-21 peptide receptor.Cell Mol Life Sci. 2021 Dec;78(23):7133-7144. doi: 10.1007/s00018-021-03944-1. Epub 2021 Oct 9. Cell Mol Life Sci. 2021. PMID: 34626205 Free PMC article. Review.
-
VGF-derived peptide TLQP-21 modulates microglial function through C3aR1 signaling pathways and reduces neuropathology in 5xFAD mice.Mol Neurodegener. 2020 Jan 10;15(1):4. doi: 10.1186/s13024-020-0357-x. Mol Neurodegener. 2020. PMID: 31924226 Free PMC article.
-
Identification of the C3a receptor (C3AR1) as the target of the VGF-derived peptide TLQP-21 in rodent cells.J Biol Chem. 2013 Sep 20;288(38):27434-27443. doi: 10.1074/jbc.M113.497214. Epub 2013 Aug 12. J Biol Chem. 2013. PMID: 23940034 Free PMC article.
-
Peptide/Receptor Co-evolution Explains the Lipolytic Function of the Neuropeptide TLQP-21.Cell Rep. 2019 Sep 3;28(10):2567-2580.e6. doi: 10.1016/j.celrep.2019.07.101. Cell Rep. 2019. PMID: 31484069 Free PMC article.
-
TLQP-21, A VGF-Derived Peptide Endowed of Endocrine and Extraendocrine Properties: Focus on In Vitro Calcium Signaling.Int J Mol Sci. 2019 Dec 24;21(1):130. doi: 10.3390/ijms21010130. Int J Mol Sci. 2019. PMID: 31878142 Free PMC article. Review.
Cited by
-
The role of complement system in adipose tissue-related inflammation.Immunol Res. 2016 Jun;64(3):653-64. doi: 10.1007/s12026-015-8783-5. Immunol Res. 2016. PMID: 26754764 Review.
-
The molecular identity of the TLQP-21 peptide receptor.Cell Mol Life Sci. 2021 Dec;78(23):7133-7144. doi: 10.1007/s00018-021-03944-1. Epub 2021 Oct 9. Cell Mol Life Sci. 2021. PMID: 34626205 Free PMC article. Review.
-
Molecular fingerprints of a convergent mechanism orchestrating diverse ligand recognition and species-specific pharmacology at the complement anaphylatoxin receptors.bioRxiv [Preprint]. 2025 May 29:2025.05.26.656101. doi: 10.1101/2025.05.26.656101. bioRxiv. 2025. PMID: 40501890 Free PMC article. Preprint.
-
TLQP-21 is a low potency partial C3aR activator on human primary macrophages.Front Immunol. 2023 Jan 26;14:1086673. doi: 10.3389/fimmu.2023.1086673. eCollection 2023. Front Immunol. 2023. PMID: 36776827 Free PMC article.
-
Complement Component C3: A Novel Biomarker Participating in the Pathogenesis of Non-alcoholic Fatty Liver Disease.Front Med (Lausanne). 2021 Jul 29;8:653293. doi: 10.3389/fmed.2021.653293. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34395461 Free PMC article. Review.
References
-
- Adessi C, Soto C. Converting a peptide into a drug: strategies to improve stability and bioavailability. Curr Med Chem. 2002;9:963–78. - PubMed
-
- Aguilar E, Pineda R, Gaytán F, Sánchez-Garrido MA, Romero M, Romero-Ruiz A, Ruiz-Pino F, Tena-Sempere M, Pinilla L. Characterization of the reproductive effects of the Vgf-derived peptide TLQP-21 in female rats: in vivo and in vitro studies. Neuroendocrinology. 2013;98:38–50. - PubMed
-
- Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W, Settmacher B, Klos A, Erhard KF, Cousins RD, et al. Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol. 2001;166:6341–8. - PubMed
-
- Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, Akerman KE, Kukkonen JP. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther. 2003;305:507–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

