Tumor necrosis factor-α-accelerated degradation of type I collagen in human skin is associated with elevated matrix metalloproteinase (MMP)-1 and MMP-3 ex vivo
- PMID: 25457675
- PMCID: PMC4300401
- DOI: 10.1016/j.ejcb.2014.10.001
Tumor necrosis factor-α-accelerated degradation of type I collagen in human skin is associated with elevated matrix metalloproteinase (MMP)-1 and MMP-3 ex vivo
Abstract
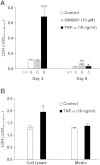
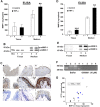
Tumor necrosis factor (TNF)-α induces matrix metalloproteinases (MMPs) that may disrupt skin integrity. We have investigated the effects and mechanisms of exogenous TNF-α on collagen degradation by incubating human skin explants in defined serum-free media with or without TNF-α (10ng/ml) in the absence or presence of the nonselective MMP inhibitor GM6001 for 8 days. The basal culture conditions promoted type I collagen catabolism that was accelerated by TNF-α (p<0.005) and accomplished by MMPs (p<0.005). Levels of the collagenases MMP-8 and MMP-13 were insignificant and neither MMP-2 nor MMP-14 were associated with increased collagen degradation. TNF-α increased secretion of MMP-1 (p<0.01) but had no impact on MMP-1 quantities in the tissue. Immunohistochemical analysis confirmed similar tissue MMP-1 expression with or without TNF-α with epidermis being the major source of MMP-1. Increased tissue-derived collagenolytic activity with TNF-α exposure was blocked by neutralizing MMP-1 monoclonal antibody and was not due to down-regulation of tissue inhibitor of metalloproteinase-1. TNF-α increased production (p<0.01), tissue levels (p<0.005) and catalytic activity of the endogenous MMP-1 activator MMP-3. Type I collagen degradation correlated with MMP-3 tissue levels (rs=0.68, p<0.05) and was attenuated with selective MMP-3 inhibitor. Type I collagen formation was down-regulated in cultured compared with native skin explants but was not reduced further by TNF-α. TNF-α had no significant effect on epidermal apoptosis. Our data indicate that TNF-α augments collagenolytic activity of MMP-1, possibly through up-regulation of MMP-3 leading to gradual loss of type I collagen in human skin.
Keywords: Aging; C-terminal telopeptide of type I collagen; Cytokine; Extracellular matrix proteins; Protease inhibitors; Type I C-terminal collagen propeptide; UK370106.
Copyright © 2014 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures







References
-
- Ågren M.S., Andersen T.L., Andersen L., Schiødt C.B., Surve V., Andreassen T.T. Nonselective matrix metalloproteinase but not tumor necrosis factor-alpha inhibition effectively preserves the early critical colon anastomotic integrity. Int. J. Colorectal Dis. 2011;26:329–337. - PubMed
-
- Ågren M.S., Andersen T.L., Mirastschijski U., Syk I., Schiødt C.B., Surve V. Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: an immunohistochemical and biochemical study. Surgery. 2006;140:72–82. - PubMed
-
- Bashir M.M., Sharma M.R., Werth V.P. TNF-alpha production in the skin. Arch. Dermatol. Res. 2009;301:87–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

