Invadosomes in their natural habitat
- PMID: 25457677
- PMCID: PMC4262535
- DOI: 10.1016/j.ejcb.2014.10.002
Invadosomes in their natural habitat
Abstract
Podosomes and invadopodia (collectively known as invadosomes) are small, F-actin-rich protrusions that are located at points of cell-ECM contacts and endow cells with invasive capabilities. So far, they have been identified in human or murine immune (myelomonocytic), vascular and cancer cells. The overarching reason for studying invadosomes is their connection to human disease. For example, macrophages and osteoclasts lacking Wiskott-Aldrich syndrome protein (WASp) are not able to form podosomes, and this leads to altered macrophage chemotaxis and defective bone resorption by osteoclasts. In contrast, the ability of cancer cells to form invadopodia is associated with high invasive and metastatic potentials. While invadosome composition, dynamics and signaling cascades leading to their assembly can be followed easily in in vitro assays, studying their contribution to pathophysiological processes in situ remains challenging. A number of recent papers have started to address this issue and describe invadosomes in situ in mouse models of cancer, cardiovascular disease and angiogenesis. In addition, in vivo invadosome homologs have been reported in developmental model systems such as C. elegans, zebrafish and sea squirt. Comparative analyses among different invasion mechanisms as they happen in their natural habitats, i.e., in situ, may provide an outline of the invadosome evolutionary history, and guide our understanding of the roles of the invasion process in pathophysiology versus development.
Keywords: Cancer; Cell locomotion; Cell migration; Cell motility; Confocal; In situ; In vivo; Invadopodia; Invadosomes; Invasion; Microenvironment; Microscopy; Multiphoton; Podosomes; Protrusions; development.
Copyright © 2014 Elsevier GmbH. All rights reserved.
Figures

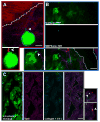
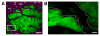


References
-
- Albinsson S, Sward K. Targeting smooth muscle microRNAs for therapeutic benefit in vascular disease. Pharmacol Res. 2013;75:28–36. - PubMed
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci. 2003;116:217–24. - PubMed
-
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–43. - PubMed
-
- Ayala I, Giacchetti G, Caldieri G, Attanasio F, Mariggiò S, Tetè S, Polishchuk R, Castronovo V, Buccione R. Faciogenital dysplasia protein Fgd1 regulates invadopodia biogenesis and extracellular matrix degradation and is up-regulated in prostate and breast cancer. Cancer Res. 69:747–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

