Ebola virus convalescent blood products: where we are now and where we may need to go
- PMID: 25457751
- PMCID: PMC7106377
- DOI: 10.1016/j.transci.2014.10.003
Ebola virus convalescent blood products: where we are now and where we may need to go
Abstract
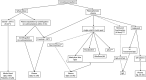
The world is regularly exposed to emerging infections with the potential to burst into a pandemic. One possible way to treat patients, when no other treatment is yet developed,is passive immunization performed by transfusing blood, plasma or plasma immunoglobul infractions obtained from convalescent donors who have recovered from the disease and have developed protective antibodies. The most recent on-going epidemic is caused by the Ebola virus, a filovirus responsible for Ebola virus disease, a severe, often lethal, hemorrhagic fever. Recently, the use of convalescent blood products was proposed by the WHO as one early option for treating patients with Ebola virus disease. This publication provides an overview of the various convalescent blood products and technological options that could theoretically be considered when there is a need to rely on this therapeutic approach.In countries without access to advanced blood-processing technologies, the choice may initially be restricted to convalescent whole blood or plasma. In technologically advanced countries, additional options for convalescent blood products are available, including virally inactivated plasma and fractionated immunoglobulins. The preparation of minipool immunoglobulins is also a realistic option to consider.
Figures
References
-
- Blajchman M.A. Protecting the blood supply from emerging pathogens: the role of pathogen inactivation. Transfus Clin Biol. 2009;16:70–74. - PubMed
-
- Wong V.W., Dai D., Wu A.K., Sung J.J. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9:199–201. - PubMed
-
- Kong L.K., Zhou B.P. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med J. 2006;12:489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

