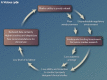
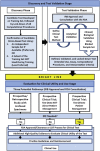
Biomarker validation and testing
- PMID: 25458054
- PMCID: PMC5528748
- DOI: 10.1016/j.molonc.2014.10.004
Biomarker validation and testing
Abstract
A tumor biomarker is a molecular or process-based change that reflects the status of an underlying malignancy. A tumor biomarker may be identified and measured by one or more assays, or tests, for the biomarker. Increasingly, tumor biomarker tests are being used to drive patient management, either by identifying patients who do not require any, or any further, treatment, or by identifying patients whose tumors are so unlikely to respond to a given type of treatment that it will cause more harm than good. A tumor biomarker assay should only be used to guide management if it has analytical validity, meaning that it is accurate, reproducible, and reliable, and if it has been shown to have clinical utility. The latter implies that high levels of evidence are available that demonstrate that application of the tumor biomarker test for a given use context results in better outcomes, or similar outcomes with less cost, than if the assay were not applied. Use contexts include risk categorization, screening, differential diagnosis, prognosis, prediction of therapeutic activity or monitoring disease course. Very few tumor biomarker tests have passed these high bars for routine clinical application. However, if tumor biomarker tests are going to be used to drive patient care, than an understanding, and careful assessment, of these concepts are essential, since "A Bad Tumor Biomarker Test Is as Bad as a Bad Drug."
Keywords: Level of evidence; Tumor biomarkers; Validation.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- AJCC, 2010. Breast. In Edge S.B., Byrd D.R., Compton C., Fritz A.G., Greene F.L., Trotti A.(Eds.), AJCC Staging Manual. Seventh ed. Springer; New York, Dordrecht, Heidelberg, London:
-
- Andre, F. , McShane, L.M. , Michiels, S. , Ransohoff, D.F. , Altman, D.G. , Reis-Filho, J.S. , Hayes, D.F. , Pusztai, L. , 2011. Biomarker studies: a call for a comprehensive biomarker study registry. Nat. Rev. Clin. Oncol. 8, 171–176. - PubMed
-
- Hammond, M.E. , Hayes, D.F. , Dowsett, M. , Allred, D.C. , Hagerty, K.L. , Badve, S. , Fitzgibbons, P.L. , Francis, G. , Goldstein, N.S. , Hayes, M. , Hicks, D.G. , Lester, S. , Love, R. , Mangu, P.B. , McShane, L. , Miller, K. , Osborne, C.K. , Paik, S. , Perlmutter, J. , Rhodes, A. , Sasano, H. , Schwartz, J.N. , Sweep, F.C. , Taube, S. , Torlakovic, E.E. , Valenstein, P. , Viale, G. , Visscher, D. , Wheeler, T. , Williams, R.B. , Wittliff, J.L. , Wolff, A.C. , 2010. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 28, 2784–2795. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials