Fast and efficient neural conversion of human hematopoietic cells
- PMID: 25458894
- PMCID: PMC4264063
- DOI: 10.1016/j.stemcr.2014.10.008
Fast and efficient neural conversion of human hematopoietic cells
Abstract
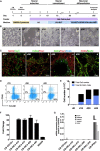
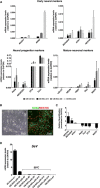
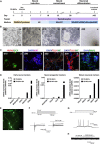
Neurons obtained directly from human somatic cells hold great promise for disease modeling and drug screening. Available protocols rely on overexpression of transcription factors using integrative vectors and are often slow, complex, and inefficient. We report a fast and efficient approach for generating induced neural cells (iNCs) directly from human hematopoietic cells using Sendai virus. Upon SOX2 and c-MYC expression, CD133-positive cord blood cells rapidly adopt a neuroepithelial morphology and exhibit high expansion capacity. Under defined neurogenic culture conditions, they express mature neuronal markers and fire spontaneous action potentials that can be modulated with neurotransmitters. SOX2 and c-MYC are also sufficient to convert peripheral blood mononuclear cells into iNCs. However, the conversion process is less efficient and resulting iNCs have limited expansion capacity and electrophysiological activity upon differentiation. Our study demonstrates rapid and efficient generation of iNCs from hematopoietic cells while underscoring the impact of target cells on conversion efficiency.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Ban H., Nishishita N., Fusaki N., Tabata T., Saeki K., Shikamura M., Takada N., Inoue M., Hasegawa M., Kawamata S., Nishikawa S. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc. Natl. Acad. Sci. USA. 2011;108:14234–14239. - PMC - PubMed
-
- Bellin M., Marchetto M.C., Gage F.H., Mummery C.L. Induced pluripotent stem cells: the new patient? Nat. Rev. Mol. Cell Biol. 2012;13:713–726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

