Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis
- PMID: 25458910
- PMCID: PMC4426225
- DOI: 10.1016/j.jaci.2014.10.001
Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis
Abstract
Background: Respiratory syncytial virus (RSV) bronchiolitis in infancy is a major risk factor for recurrent wheezing and asthma. Because azithromycin attenuated neutrophilic airway inflammation in a murine viral bronchiolitis model, demonstration of similar effects in human subjects might provide a strategy for the prevention of postbronchiolitis recurrent wheezing.
Objectives: We sought to investigate whether azithromycin treatment during RSV bronchiolitis reduces serum and nasal lavage IL-8 levels and the occurrence of postbronchiolitis recurrent wheezing.
Method: We performed a randomized, double-masked, placebo-controlled proof-of-concept trial in 40 otherwise healthy infants hospitalized with RSV bronchiolitis who were treated with azithromycin or placebo for 14 days. IL-8 levels were measured in nasal lavage fluid and serum on randomization, day 8, and day 15 (nasal lavage only). The occurrence of wheezing episodes was assessed monthly over the ensuing 50 weeks.
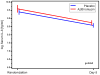
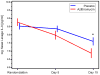
Results: Compared with placebo, azithromycin treatment did not reduce serum IL-8 levels at day 8 (P = .6) but resulted in a greater decrease in nasal lavage fluid IL-8 levels by day 15 (P = .03). Twenty-two percent of azithromycin-treated participants experienced at least 3 wheezing episodes compared with 50% of participants in the placebo group (P = .07). Azithromycin treatment resulted in prolonged time to the third wheezing episode (P = .048) and in fewer days with respiratory symptoms over the subsequent year in comparison with placebo (36.7 vs 70.1 days, P = .01).
Conclusion: In this proof-of-concept study azithromycin treatment during RSV bronchiolitis reduced upper airway IL-8 levels, prolonged the time to the third wheezing episode, and reduced overall respiratory morbidity over the subsequent year.
Keywords: Azithromycin; IL-8; respiratory syncytial virus; wheezing.
Copyright © 2014 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Reply: To PMID 25458910.J Allergy Clin Immunol. 2015 Oct;136(4):1135-6. doi: 10.1016/j.jaci.2015.07.013. Epub 2015 Aug 22. J Allergy Clin Immunol. 2015. PMID: 26309182 No abstract available.
-
Further clinical trials on macrolides for bronchiolitis in infants are unnecessary.J Allergy Clin Immunol. 2015 Oct;136(4):1134-5. doi: 10.1016/j.jaci.2015.07.012. Epub 2015 Aug 24. J Allergy Clin Immunol. 2015. PMID: 26316096 No abstract available.
References
-
- Simoes EA. Respiratory syncytial virus infection. Lancet. 1999;354:847–52. - PubMed
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. American journal of diseases of children. 1986;140:543–6. - PubMed
-
- Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J. 2002;21:629–32. - PubMed
-
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143:S127–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

