Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner
- PMID: 25459519
- PMCID: PMC4371601
- DOI: 10.1016/j.alcohol.2014.07.015
Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner
Abstract
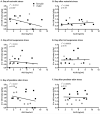
Exposure to stress contributes to ethanol consumption in humans, but it produces inconsistent effects on ethanol drinking in rodent models. Therefore, the present study examined the influence of different stressors (restraint, tail suspension, predator odor, foot shock, and tail pinch) on 2-h access to water and 10% ethanol by male and female C57BL/6J mice and determined whether there were sex-dependent differences in response to stress. Plasma corticosterone (CORT) and allopregnanolone (ALLO) were assessed as indexes of hypothalamic-pituitary-adrenal axis activity and of endogenous neurosteroid levels, respectively, following restraint, tail suspension, and predator odor. These stressors increased plasma CORT and ALLO levels, and produced a greater increase in CORT and ALLO levels in females versus males. Ethanol intake was decreased following restraint, tail suspension, foot shock, and tail pinch in both sexes, with stressor-related differences in the duration of the suppression. Predator odor significantly increased ethanol intake on the following two days in females and on the second day after stress in males. Notably, there was a significant positive correlation between CORT levels immediately after predator odor stress and ethanol intake on the following day. In summary, the type of stressor influenced ethanol consumption, with subtle sex differences in the magnitude and persistence of the effect. These findings are the first to demonstrate that a single, acute exposure to restraint, tail suspension, and predator odor stress increased plasma CORT and ALLO levels in animals with a history of ethanol consumption and that female mice were more responsive than males to the ability of stress to increase CORT and ALLO levels as well as to increase ethanol intake following predator odor stress. Because predator odor stress is a model of post-traumatic stress disorder (PTSD), the present sex differences have important implications for preclinical studies modeling the comorbidity of PTSD and alcohol use disorders.
Keywords: Allopregnanolone; Corticosterone; Environmental stress; Foot shock; Predator odor; Tail pinch.
Published by Elsevier Inc.
Figures

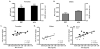
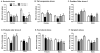
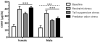

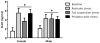
References
-
- Adams N. Sex differences and the effects of tail pinch on ethanol drinking in Maudsley rats. Alcohol. 1995;12:463–468. - PubMed
-
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci. Biobehav. Rev. 2005;29:525–546. - PubMed
-
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int. Rev. Neurobiol. 2001;46:243–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

