The azaindole framework in the design of kinase inhibitors
- PMID: 25460315
- PMCID: PMC6271083
- DOI: 10.3390/molecules191219935
The azaindole framework in the design of kinase inhibitors
Abstract
This review article illustrates the growing use of azaindole derivatives as kinase inhibitors and their contribution to drug discovery and innovation. The different protein kinases which have served as targets and the known molecules which have emerged from medicinal chemistry and Fragment-Based Drug Discovery (FBDD) programs are presented. The various synthetic routes used to access these compounds and the chemical pathways leading to their synthesis are also discussed. An analysis of their mode of binding based on X-ray crystallography data gives structural insights for the design of more potent and selective inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

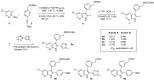

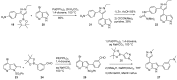

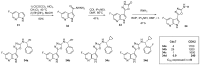
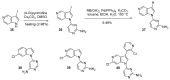

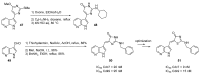
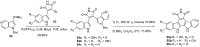


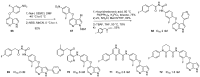
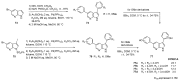



















Similar articles
-
N-substituted azaindoles as potent inhibitors of Cdc7 kinase.Bioorg Med Chem Lett. 2013 Apr 1;23(7):2056-60. doi: 10.1016/j.bmcl.2013.02.007. Epub 2013 Feb 13. Bioorg Med Chem Lett. 2013. PMID: 23481650
-
ROCK inhibitors 3: Design, synthesis and structure-activity relationships of 7-azaindole-based Rho kinase (ROCK) inhibitors.Bioorg Med Chem Lett. 2018 Aug 15;28(15):2622-2626. doi: 10.1016/j.bmcl.2018.06.040. Epub 2018 Jun 19. Bioorg Med Chem Lett. 2018. PMID: 30082069
-
Janus kinase 2 inhibitors. Synthesis and characterization of a novel polycyclic azaindole.J Med Chem. 2009 Dec 24;52(24):7938-41. doi: 10.1021/jm901383u. J Med Chem. 2009. PMID: 20014869
-
7-Azaindole: A Versatile Scaffold for Developing Kinase Inhibitors.Chem Pharm Bull (Tokyo). 2018;66(1):29-36. doi: 10.1248/cpb.c17-00380. Chem Pharm Bull (Tokyo). 2018. PMID: 29311509 Review.
-
The importance of indole and azaindole scaffold in the development of antitumor agents.Eur J Med Chem. 2020 Oct 1;203:112506. doi: 10.1016/j.ejmech.2020.112506. Epub 2020 Jun 28. Eur J Med Chem. 2020. PMID: 32688198 Review.
Cited by
-
A highly efficient precatalytic system (XPhos-PdG2) for the Suzuki-Miyaura cross-coupling of 7-chloro-1H-pyrrolo[2,3-c]pyridine employing low catalyst loading.Mol Divers. 2019 Aug;23(3):697-707. doi: 10.1007/s11030-018-9904-6. Epub 2019 Jan 9. Mol Divers. 2019. PMID: 30627855
-
Understanding and Interrupting the Fischer Azaindolization Reaction.J Am Chem Soc. 2017 Oct 25;139(42):14833-14836. doi: 10.1021/jacs.7b07518. Epub 2017 Oct 12. J Am Chem Soc. 2017. PMID: 29022706 Free PMC article.
-
Structure-Activity Relationship (SAR) Study of Spautin-1 to Entail the Discovery of Novel NEK4 Inhibitors.Int J Mol Sci. 2021 Jan 10;22(2):635. doi: 10.3390/ijms22020635. Int J Mol Sci. 2021. PMID: 33435251 Free PMC article.
-
Levoglucosenone and Its Pseudoenantiomer iso-Levoglucosenone as Scaffolds for Drug Discovery and Development.ACS Omega. 2020 Jun 8;5(23):13926-13939. doi: 10.1021/acsomega.0c01331. eCollection 2020 Jun 16. ACS Omega. 2020. PMID: 32566859 Free PMC article.
-
Deprotometalation-Iodolysis and Direct Iodination of 1-Arylated 7-Azaindoles: Reactivity Studies and Molecule Properties.Molecules. 2021 Oct 19;26(20):6314. doi: 10.3390/molecules26206314. Molecules. 2021. PMID: 34684895 Free PMC article.
References
-
- Yang S.-W., Abdel-Kader M., Malone S., Werkhoven M.C.M., Wisse J.H., Bursuker I., Neddermann K., Fairchild C., Raventos-Suarez C., Menendez A.T., et al. Synthesis and Biological Evaluation of Analogues of Cryptolepine, an Alkaloid Isolated from the Suriname Rainforest1. J. Nat. Prod. 1999;62:976–983. doi: 10.1021/np990035g. - DOI - PubMed
-
- Paulo A., Gomes E.T., Houghton P.J. New Alkaloids from Cryptolepis sanguinolenta. J. Nat. Prod. 1995;58:1485–1491. doi: 10.1021/np50124a002. - DOI
-
- Choshi T., Yamada S., Sugino E., Kuwada T., Hibino S. Total synthesis of grossularines-1 and -2. J. Org. Chem. 1995;60:5899–5904. doi: 10.1021/jo00123a028. - DOI
-
- Simone M., Erba E., Damia G., Vikhanskaya F., di Francesco A.M., Riccardi R., Bailly C., Cuevas C., Fernandez Sousa-Faro J.M., D’Incalci M. Variolin B and its derivate deoxy-variolin B: New marine natural compounds with cyclin-dependent kinase inhibitor activity. Eur. J. Cancer Oxf. Engl. 1990. 2005;41:2366–2377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

