Simulation study for evaluating the performance of response-adaptive randomization
- PMID: 25460340
- PMCID: PMC4314433
- DOI: 10.1016/j.cct.2014.11.006
Simulation study for evaluating the performance of response-adaptive randomization
Abstract
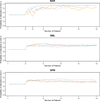
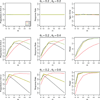
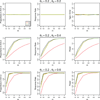
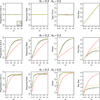
A response-adaptive randomization (RAR) design refers to the method in which the probability of treatment assignment changes according to how well the treatments are performing in the trial. Holding the promise of treating more patients with the better treatments, RARs have been successfully implemented in clinical trials. We compared equal randomization (ER) with three RARs: Bayesian adaptive randomization, sequential maximum likelihood, and sequential posterior mean. We fixed the total number of patients, considered as patient horizon, but varied the number of patients in the trial. Among the designs, we compared the proportion of patients assigned to the superior arm, overall response rate, statistical power, and total patients enrolled in the trial with and without adding an efficacy early stopping rule. Without early stopping, ER is preferred when the number of patients beyond the trial is much larger than the number of patients in the trial. RAR is favored for large treatment difference or when the number of patients beyond the trial is small. With early stopping, the difference between these two types of designs was reduced. By carefully choosing the design parameters, both RAR and ER methods can achieve the desirable statistical properties. Within three RAR methods, we recommend SPM considering the larger proportion in the better arm and higher overall response rate than BAR and similar power and trial size with ER. The ultimate choice of RAR or ER methods depends on the investigator's preference, the trade-off between group ethics and individual ethics, and logistic considerations in the trial conduct, etc.
Keywords: Allocation probability; Bayesian adaptive design; Efficacy early stopping; Operating characteristics; Patient horizon.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
Comment in
-
A note on response-adaptive randomization.Contemp Clin Trials. 2015 Jan;40:240. doi: 10.1016/j.cct.2014.12.013. Epub 2014 Dec 26. Contemp Clin Trials. 2015. PMID: 25545024 No abstract available.
References
-
- Berry DA. Bayesian Statistics and the Efficiency and Ethics of Clinical Trials. Statistical Science. 2004;19:175–187.
-
- Zelen M. Play the winner rule and the controlled clinical trial. Journal of the American Statistical Association. 1969;64:131–146.
-
- Wei LJ. An application of an urn model to the design of sequential controlled clinical trials. Journal of the American Statistical Association. 1978;73:559–563.
-
- Rosenberger WF, Lachin JM. Randomization in Clinical Trials: Theory and Practice. New York: John Wiley and Sons; 2002.
-
- Hu FF, Zhang LX. Asymptotic properties of double adaptive biased coin designs for multi-treatment clinical trials. Annals of Statistics. 2004;32:268–301.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

