The Masquelet technique for membrane induction and the healing of ovine critical sized segmental defects
- PMID: 25461340
- PMCID: PMC4252083
- DOI: 10.1371/journal.pone.0114122
The Masquelet technique for membrane induction and the healing of ovine critical sized segmental defects
Abstract
The healing of critical sized segmental defects is an ongoing clinical problem. No method has achieved pre-eminence. The Masquelet technique is a relatively new innovation involving the induction of a fibrous tissue membrane around the bone defect site taking advantage of the body's foreign body reaction to the presence of a polymethylmethacrylate (PMMA) spacer. The aim of this study was to investigate the properties and characteristics of this induced membrane and its effectiveness when used in conjunction with allograft or an allograft/autograft mix as filler materials in an ovine critical sized defect model. The resultant induced membrane was found to be effective in containing the graft materials in situ. It was demonstrated to be an organised pseudosynovial membrane which expressed bone morphogenic protein 2 (BMP2), transforming growth factor-beta (TGFβ), vascular endothelial growth factor (VEGF), von Willerbrand factor (vWF), interleukin 6 (IL-6) and interleukin 8 (IL-8). While more new bone growth was evident in the test groups compared to the controls animals at 12 weeks, the volumes were not statistically different and no defects were fully bridged. Of the two graft material groups, the allograft/autograft mix was shown to have a more rapid graft resorption rate than the allograft only group. While the Masquelet technique proved effective in producing a membrane to enclose graft materials, its ability to assist in the healing of critical sized segmental defects when compared to empty controls remained inconclusive.
Conflict of interest statement
Figures
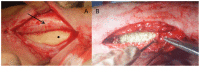






References
-
- Stafford PR, Norris BL (2010) Reamer-irrigator-aspirator bone graft and bi Masquelet technique for segmental bone defect nonunions: a review of 25 cases. Injury 41 Suppl 2: S72–77. - PubMed
-
- Giannoudis PV, Dinopoulos H, Tsiridis E (2005) Bone substitutes: an update. Injury 36 Suppl 3: S20–27. - PubMed
-
- Pluhar GE, Turner AS, Pierce AR, Toth CA, Wheeler DL (2006) A comparison of two biomaterial carriers for osteogenic protein-1 (BMP-7) in an ovine critical defect model. J Bone Joint Surg Br 88:960–966. - PubMed
-
- Govender SM, Csimma CRM, Genant HKM, Valentin-Opran AM, The Bmp-2 Evaluation In Surgery For Tibial Trauma Study G (2002) Recombinant Human Bone Morphogenetic Protein-2 For Treatment Of Open Tibial Fractures: A Prospective, Controlled, Randomized Study Of Four Hundred And Fifty Patients. Journal of Bone & Joint Surgery - American Volume 84-A:2123–2134. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

