Neutrophil serine proteases in antibacterial defense
- PMID: 25461571
- PMCID: PMC4323955
- DOI: 10.1016/j.mib.2014.11.002
Neutrophil serine proteases in antibacterial defense
Abstract
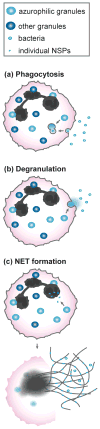
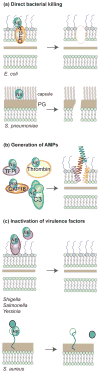
Neutrophil serine proteases (NSPs) are critical for the effective functioning of neutrophils and greatly contribute to immune protection against bacterial infections. Thanks to their broad substrate specificity, these chymotrypsin-like proteases trigger multiple reactions that are detrimental to bacterial survival such as direct bacterial killing, generation of antimicrobial peptides, inactivation of bacterial virulence factors and formation of neutrophil extracellular traps. Recently, the importance of NSPs in antibacterial defenses has been further underscored by discoveries of unique bacterial evasion strategies to combat these proteases. Bacteria can indirectly disarm NSPs by protecting bacterial substrates against NSP cleavage, but also produce inhibitory molecules that potently block NSPs. Here we review recent insights in the functional contribution of NSPs in host protection against bacterial infections and the elegant strategies that bacteria use to counteract these responses.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. - PubMed
-
- Reeves EP, Lu H, Jacobs HL, Messina CGM, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

