TCR-mediated functions are enhanced in activated peripheral blood T cells isolated from leucocyte reduction systems
- PMID: 25462023
- PMCID: PMC4324009
- DOI: 10.1016/j.jim.2014.11.009
TCR-mediated functions are enhanced in activated peripheral blood T cells isolated from leucocyte reduction systems
Abstract
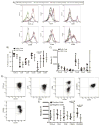
Buffy coats are the most common method for the acquisition of activated primary human T cells for research or clinical applications, but recently leukocyte reduction system (LRS) cones have emerged as a viable source for these cells. In this study, we determined if activated human T cells derived from buffy coats or LRS cones had different functionality. No changes in the expression of surface receptors were observed except for a significant increase in CD44 expression on T cells isolated from LRS cones. LRS cone-derived T cells trended towards higher receptor-mediated cytokine production and had significantly increased donor-to-donor variability in IFN-γ production. TCR-induced ERK1/ERK2 and AKT phosphorylation was also increased in T cells isolated from LRS cones. In conclusion, LRS cones are an excellent source of T cells for clinical and research applications, but these cells have subtle functional differences from T cells isolated using standard buffy coats.
Keywords: Buffy coat; Leukocyte reduction systems; T cell receptor; T cells.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
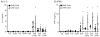

Similar articles
-
Filter Buffy Coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters.J Immunol Methods. 2005 Dec 20;307(1-2):150-66. doi: 10.1016/j.jim.2005.10.004. Epub 2005 Nov 17. J Immunol Methods. 2005. PMID: 16325197
-
Alpha2 beta1 integrin signaling augments T cell receptor-dependent production of interferon-gamma in human T cells.Mol Immunol. 2007 Jul;44(15):3732-40. doi: 10.1016/j.molimm.2007.04.003. Epub 2007 May 22. Mol Immunol. 2007. PMID: 17521731
-
Enhancement of T cell responses as a result of synergy between lower doses of radiation and T cell stimulation.J Immunol. 2014 Apr 1;192(7):3101-10. doi: 10.4049/jimmunol.1302736. Epub 2014 Mar 5. J Immunol. 2014. PMID: 24600032
-
Mycobacterial antigen(s) induce anergy by altering TCR- and TCR/CD28-induced signalling events: insights into T-cell unresponsiveness in leprosy.Mol Immunol. 2010 Feb;47(5):943-52. doi: 10.1016/j.molimm.2009.11.009. Epub 2009 Dec 16. Mol Immunol. 2010. PMID: 20018378
-
Leukocyte reduction filters as an alternative source of peripheral blood leukocytes for research.Hematol Transfus Cell Ther. 2021 Oct-Dec;43(4):494-498. doi: 10.1016/j.htct.2020.10.963. Epub 2020 Dec 24. Hematol Transfus Cell Ther. 2021. PMID: 33422490 Free PMC article. Review.
Cited by
-
Exposure of Human CD4 T Cells to IL-12 Results in Enhanced TCR-Induced Cytokine Production, Altered TCR Signaling, and Increased Oxidative Metabolism.PLoS One. 2016 Jun 9;11(6):e0157175. doi: 10.1371/journal.pone.0157175. eCollection 2016. PLoS One. 2016. PMID: 27280403 Free PMC article.
-
Optimization of methods for the genetic modification of human T cells.Immunol Cell Biol. 2015 Nov;93(10):896-908. doi: 10.1038/icb.2015.59. Epub 2015 Jun 1. Immunol Cell Biol. 2015. PMID: 26027856 Free PMC article.
-
A Novel Approach on Leukodepletion Filters: Investigation of Synergistic Anticancer Effect of Purified α-Defensins and Nisin.Adv Pharm Bull. 2021 Feb;11(2):378-384. doi: 10.34172/apb.2021.036. Epub 2020 Apr 19. Adv Pharm Bull. 2021. PMID: 33880361 Free PMC article.
-
Platelet apheresis with additive solution and plasma rinseback affects the cellular composition of LRS chamber products.Sci Rep. 2025 Jun 6;15(1):19923. doi: 10.1038/s41598-025-04350-4. Sci Rep. 2025. PMID: 40481081 Free PMC article.
-
Pretreatment of activated human CD8 T cells with IL-12 leads to enhanced TCR-induced signaling and cytokine production.Mol Immunol. 2017 Jan;81:1-15. doi: 10.1016/j.molimm.2016.11.008. Epub 2016 Nov 22. Mol Immunol. 2017. PMID: 27883938 Free PMC article.
References
-
- Shi H, Liu L, Wang Z. Improving the efficacy and safety of engineered T cell therapy for cancer. Cancer Lett. 2013;328:191–197. - PubMed
-
- Ebner S, Neyer S, Hofer S, Nussbaumer W, Romani N, Heufler C. Generation of large numbers of human dendritic cells from whole blood passaged through leukocyte removal filters: an alternative to standard buffy coats. J Immunol Methods. 2001;252:93–104. - PubMed
-
- Meyer TP, Zehnter I, Hofmann B, Zaisserer J, Burkhart J, Rapp S, Weinauer F, Schmitz J, Illert WE. Filter Buffy Coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. J Immunol Methods. 2005;307:150–166. - PubMed
-
- Neron S, Dussault N, Racine C. Whole-blood leukoreduction filters are a source for cryopreserved cells for phenotypic and functional investigations on peripheral blood lymphocytes. Transfusion. 2006;46:537–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

