A review on recent developments of indole-containing antiviral agents
- PMID: 25462257
- PMCID: PMC7115707
- DOI: 10.1016/j.ejmech.2014.10.065
A review on recent developments of indole-containing antiviral agents
Abstract
Indole represents one of the most important privileged scaffolds in drug discovery. Indole derivatives have the unique property of mimicking the structure of peptides and to bind reversibly to enzymes, which provide tremendous opportunities to discover novel drugs with different modes of action. There are seven indole-containing commercial drugs in the Top-200 Best Selling Drugs by US Retail Sales in 2012. There are also an amazing number of approved indole-containing drugs in the market as well as compounds currently going through different clinical phases or registration statuses. This review focused on the recent development of indole derivatives as antiviral agents with the following objectives: 1) To present one of the most comprehensive listings of indole antiviral agents, drugs on market or compounds in clinical trials; 2) To focus on recent developments of indole compounds (including natural products) and their antiviral activities, summarize the structure property, hoping to inspire new and even more creative approaches; 3) To offer perspectives on how indole scaffolds as a privileged structure might be exploited in the future.
Keywords: Antiviral activity; Entry and fusion inhibitor; Indole; Integrase inhibitor; Natural product; Polymerase inhibitor; Protease inhibitor; Reverse transcriptase inhibitor.
Copyright © 2014 Elsevier Masson SAS. All rights reserved.
Figures















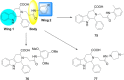














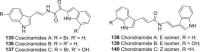
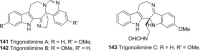
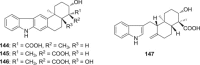



References
-
- Samala S., Arigela R.K., Kant R., Kundu B. Diversity-oriented synthesis of ketoindoloquinoxalines and indolotriazoloquinoxalines from 1-(2-nitroaryl)-2-alkynylindoles. J. Org. Chem. 2014;79:2491–2500. - PubMed
-
- Zhang M.Z., Mulholland N., Beattie D., Irwin D., Gu Y.C., Chen Q., Yang G.F., Clough J. Synthesis and antifungal activity of 3-(1,3,4-oxadiazol-5-yl)-indoles and 3-(1,3,4-oxadiazol-5-yl)methyl-indoles. Eur. J. Med. Chem. 2013;63:22–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

