Low-level light therapy potentiates NPe6-mediated photodynamic therapy in a human osteosarcoma cell line via increased ATP
- PMID: 25462575
- PMCID: PMC4361381
- DOI: 10.1016/j.pdpdt.2014.10.009
Low-level light therapy potentiates NPe6-mediated photodynamic therapy in a human osteosarcoma cell line via increased ATP
Abstract
Background: Low-level light therapy (LLLT) is used to stimulate healing, reduce pain and inflammation, and preserve tissue from dying. LLLT has been shown to protect cells in culture from dying after various cytotoxic insults, and LLLT is known to increase the cellular ATP content. Previous studies have demonstrated that maintaining a sufficiently high ATP level is necessary for the efficient induction and execution of apoptosis steps after photodynamic therapy (PDT).
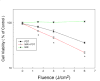
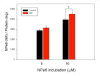
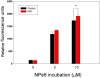
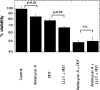
Methods: We asked whether LLLT would protect cells from cytotoxicity due to PDT, or conversely whether LLLT would enhance the efficacy of PDT mediated by mono-l-aspartyl chlorin(e6) (NPe6). Increased ATP could lead to enhanced cell uptake of NPe6 by the energy dependent process of endocytosis, and also to more efficient apoptosis. In this study, human osteosarcoma cell line MG-63 was subjected to 1.5J/cm(2) of 810nm near infrared radiation (NIR) followed by addition of 10μM NPe6 and after 2h incubation by 1.5J/cm(2) of 652nm red light for PDT.
Results: PDT combined with LLLT led to higher cell death and increased intracellular reactive oxygen species compared to PDT alone. The uptake of NPe6 was moderately increased by LLLT, and cellular ATP was increased. The mitochondrial respiratory chain inhibitor antimycin A abrogated the LLLT-induced increase in cytotoxicity.
Conclusions: Taken together, these results demonstrate that LLLT potentiates NPe6-mediated PDT via increased ATP synthesis and is a potentially promising strategy that could be applied in clinical PDT.
Keywords: Adenosine triphosphate; Low level light therapy; Lysosomal uptake; Mono-l-aspartyl chlorin(e6); Photobiomodulation; Photodynamic therapy.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

