Efficient in vitro refolding and functional characterization of recombinant human liver carboxylesterase (CES1) expressed in E. coli
- PMID: 25462813
- PMCID: PMC4294421
- DOI: 10.1016/j.pep.2014.11.006
Efficient in vitro refolding and functional characterization of recombinant human liver carboxylesterase (CES1) expressed in E. coli
Abstract
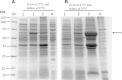
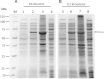
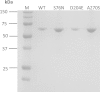
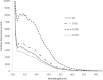
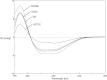
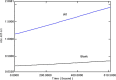
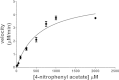
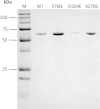
Human liver carboxylesterase 1 (CES1) plays a critical role in the hydrolysis of various ester- and amide-containing molecules, including active metabolites, drugs and prodrugs. However, it has been problematic to express recombinant CES1 in bacterial expression systems due to low solubility, with the CES1 protein being mainly expressed in inclusion bodies, accompanied by insufficient purity issues. In this study, we report an efficient in vitro method for refolding recombinant CES1 from inclusion bodies. A one-step purification with an immobilized-metal affinity column was utilized to purify His-tagged recombinant CES1. Conveniently, both denaturant and imidazole can be removed while the enzyme is refolded via buffer exchange, a dilution method. We show that the refolding of recombinant CES1 was successful in Tris-HCl at pH 7.5 containing a combination of 1% glycerol and 2 mM β-mercaptoethanol, whereas a mixture of other additives (trehalose, sorbitol and sucrose) and β-mercaptoethanol failed to recover a functional protein. His-tagged recombinant CES1 retains its biological activity after refolding and can be used directly without removing the fusion tag. Altogether, our results provide an alternative method for obtaining a substantial amount of functionally active protein, which is advantageous for further investigations such as structural and functional studies.
Keywords: Carboxylesterases; E. coli; Glycerol; Inclusion bodies; Refolding.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Laizure S.C., Mandrell T., Gades N.M., Parker R.B. Cocaethylene metabolism and interaction with cocaine and ethanol: role of carboxylesterases. Drug Metab. Dispos. 2003;31:16–20. - PubMed
-
- Tang M., Mukundan M., Yang J., Charpentier N., LeCluyse E.L., Black C., Yang D., Shi D., Yan B. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. J. Pharmacol. Exp. Ther. 2006;319:1467–1476. - PubMed
-
- Shi D., Yang J., Yang D., LeCluyse E.L., Black C., You L., Akhlaghi F., Yan B. Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel. J. Pharmacol. Exp. Ther. 2006;319:1477–1484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

