Zebrafish calsyntenins mediate homophilic adhesion through their amino-terminal cadherin repeats
- PMID: 25463516
- PMCID: PMC4298480
- DOI: 10.1016/j.neuroscience.2014.11.030
Zebrafish calsyntenins mediate homophilic adhesion through their amino-terminal cadherin repeats
Abstract
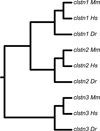
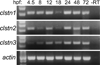
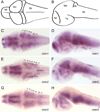
The calsyntenins are atypical members of the cadherin superfamily that have been implicated in learning in Caenorhabditis elegans and memory formation in humans. As members of the cadherin superfamily, they could mediate cell-cell adhesion, although their adhesive properties have not been investigated. As an initial step in characterizing the calsyntenins, we have cloned clstn1, clstn2 and clstn3 from the zebrafish and determined their expression in the developing zebrafish nervous system. The three genes each have broad, yet distinct, expression patterns in the zebrafish brain. Each of the ectodomains mediates homophilic interactions through two, amino-terminal cadherin repeats. In bead sorting assays, the calsyntenin ectodomains do not exhibit homophilic preferences. These data support the idea that calsyntenins could either act as adhesion molecules or as diffusible, homophilic or heterophilic ligands in the vertebrate nervous system.
Keywords: adhesion; cadherin; calsyntenin; zebrafish.
Copyright © 2014 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures




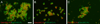
References
-
- Araki Y, Miyagi N, Kato N, Yoshida T, Wada S, Nishimura M, Komano H, Yamamoto T, De Strooper B, Yamamoto K, Suzuki T. Coordinated metabolism of Alcadein and amyloid beta-protein precursor regulates FE65-dependent gene transactivation. J Biol Chem. 2004;279:24343–24354. - PubMed
-
- Araki Y, Tomita S, Yamaguchi H, Miyagi N, Sumioka A, Kirino Y, Suzuki T. Novel cadherin-related membrane proteins, Alcadeins, enhance the X11-like protein-mediated stabilization of amyloid beta-protein precursor metabolism. J Biol Chem. 2003;278:49448–49458. - PubMed
-
- Blevins CJ, Emond MR, Biswas S, Jontes JD. Differential expression, alternative splicing, and adhesive properties of the zebrafish delta1-protocadherins. Neuroscience. 2011;199:523–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

