Antidepressants for people with epilepsy and depression
- PMID: 25464360
- PMCID: PMC6769156
- DOI: 10.1002/14651858.CD010682.pub2
Antidepressants for people with epilepsy and depression
Update in
-
Antidepressants for people with epilepsy and depression.Cochrane Database Syst Rev. 2021 Apr 16;4(4):CD010682. doi: 10.1002/14651858.CD010682.pub3. Cochrane Database Syst Rev. 2021. PMID: 33860531 Free PMC article.
Abstract
Background: Depressive disorders are the most common psychiatric comorbidity in patients with epilepsy, affecting around one-third, with a significant negative impact on quality of life. There is concern that patients may not be receiving appropriate treatment for their depression because of uncertainty regarding which antidepressant or class works best and the perceived risk of exacerbating seizures. This review aims to address these issues and inform clinical practice and future research.
Objectives: We aimed to review and synthesise evidence from randomised controlled trials of antidepressants and prospective non-randomised studies of antidepressants used for treating depression in patients with epilepsy. The primary objectives were to evaluate the efficacy and safety of antidepressants in treating depressive symptoms and the effect on seizure recurrence.
Search methods: We conducted a search of the following databases: the Cochrane Epilepsy Group Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 5), MEDLINE (Ovid), SCOPUS, PsycINFO, www.clinicaltrials.gov and conference proceedings, including studies published up to 31 May 2014. There were no language restrictions.
Selection criteria: We included randomised controlled trials (RCTs) and prospective non-randomised cohort controlled and uncontrolled studies investigating children or adults with epilepsy treated with an antidepressant for depressive symptoms. The intervention group consisted of patients receiving an antidepressant drug in addition to an existing antiepileptic drug regimen. The control group(s) consisted of patients receiving a placebo, comparative antidepressant, psychotherapy or no treatment in addition to an existing antiepileptic drug regimen.
Data collection and analysis: We extracted data on trial design factors, patient demographics and outcomes for each study. The primary outcomes were changes in depression scores (proportion with a greater than 50% improvement or mean difference) and change in seizure frequency (mean difference or proportion with a seizure recurrence or episode of status epilepticus, or both). Secondary outcomes included the number of patients withdrawing from the study and reasons for withdrawal, as well as any adverse events. Two authors undertook data extraction separately for each included study. We then cross-checked the data extraction. We assessed risk of bias using a version of the extended Cochrane Collaboration tool for assessing risk of bias in both randomised and non-randomised studies. We presented binary outcomes as risk ratios (RRs) with 95% confidence intervals (CIs). We presented continuous outcomes as standardised mean differences (SMDs) with 95% CIs, and mean differences (MDs) with 95% CIs. If possible we intended to use meta-regression techniques to investigate possible sources of heterogeneity however this was not possible due to lack of data.
Main results: We included in the review eight studies (three RCTs and five prospective cohort studies) including 471 patients with epilepsy treated with an antidepressant. The RCTs were all single-centre studies comparing an antidepressant versus active control, placebo or no treatment. The five non-randomised prospective cohort studies reported on outcomes mainly in patients with partial epilepsy treated for depression with a selective serotonin reuptake inhibitor (SSRI). We rated all the RCTs and one prospective cohort study as having unclear risk of bias. We rated the four other prospective cohort studies as having high risk of bias. We were unable to perform any meta-analysis for the proportion with a greater than 50% improvement in depression scores because the studies reported on different treatment comparisons. The results are presented descriptively and show a varied responder rate of between 24% and 97%, depending on the antidepressant given. For the mean difference in depression score we were able to perform a limited meta-analysis of two prospective cohort studies of citalopram, including a total of 88 patients. This gave low quality evidence for the effect estimate of 1.17 (95% CI 0.96 to 1.38) in depression scores. Seizure frequency data were not reported in any RCTs and we were unable to perform any meta-analysis for prospective cohort studies due to the different treatment comparisons. The results are presented descriptively and show that treatment in three studies with a selective serotonin reuptake inhibitor did not significantly increase seizure frequency. Patients given an antidepressant were more likely to withdraw due to adverse events than inefficacy. Reported adverse events for SSRIs included nausea, dizziness, sedation, gastrointestinal disturbance and sexual dysfunction. Across three comparisons we rated the evidence as moderate quality due to the small sizes of the contributing studies and only one study each contributing to the comparisons. We rated the evidence for the final comparison as low quality as there was concern over the study methods in the two contributing studies.
Authors' conclusions: Existing evidence on the effectiveness of antidepressants in treating depressive symptoms associated with epilepsy is very limited. Only one small RCT demonstrated a statistically significant effect of venlafaxine on depressive symptoms. We have no high quality evidence to inform the choice of antidepressant drug or class of drug in treating depression in people with epilepsy. This review provides low quality evidence of safety in terms of seizure exacerbation with SSRIs, but there are no available comparative data on antidepressant classes and safety in relation to seizures. There are currently no comparative data on antidepressants and psychotherapy in treating depression in epilepsy, although psychotherapy could be considered in patients unwilling to take antidepressants or where there are unacceptable side effects. Further comparative clinical trials of antidepressants and psychotherapy in large cohorts of patients with epilepsy and depression are required to better inform treatment policy in the future.
Conflict of interest statement
MM, JP, JS and AM have no declarations of interest.
Figures

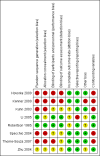









References
References to studies included in this review
-
- Hovorka J, Herman E, Nemcova I. Treatment of interictal depression with citalopram in patients with epilepsy. Epilepsy and Behavior 2000;1:444‐7. - PubMed
-
- Kanner A, Kozak A, Frey M. The use of sertraline in patients with epilepsy: is it safe?. Epilepsy and Behavior 2000;1:100‐5. - PubMed
-
- Kuhn K, Quednow B, Thiel M, Falkai P, Maier W, Elger C. Antidepressive treatment in patients with temporal lobe epilepsy and major depression: a prospective study with three different antidepressants. Epilepsy and Behavior 2003;4:674‐9. - PubMed
-
- Li W, Ma D. A randomized controlled trial to evaluate the efficacy of paroxetine and doxepin in treating epileptic patients with depression. Chinese Journal of Clinical Rehabilitation 2005;9(12):20‐1.
-
- Robertson M, Trimble M. The treatment of depression in patients with epilepsy: a double blind trial. Journal of Affective Disorders 1985;9:127‐36. - PubMed
References to studies excluded from this review
-
- Blumer D. Antidepressant and double antidepressant treatment for the affective disorder of epilepsy. Journal of Clinical Psychiatry 1997;58(1):3‐11. - PubMed
-
- Gilliam F. Depression and health outcomes in refractory epilepsy. www.clinicaltrials.gov/ct/show/NCT000266372005.
-
- Kocsis. Lexapro for major depression in patients with epilepsy. www.clinicaltrials.gov/ct/show/NCT012447242007.
References to studies awaiting assessment
-
- Conrad E. Escitalopram treatment of major depression in patients with temporal lobe epilepsy. www.clinicaltrials.gov/ct/show/NCT005956992008.
-
- Harmant J, Rijckevorsel‐Harmant K, Barsy T, Hendrickx B. Fluvoxamine: an antidepressant with low (or no) epileptogenic effect. Lancet 1990;336(8711):386. - PubMed
-
- Machado R, Espinosa A, Montoto A. Cholesterol concentrations and clinical response to sertraline in patients with epilepsy: preliminary results. Epilepsy & Behavior 2010;19:509‐12. - PubMed
Additional references
-
- Adams SJ, O'Brien TJ, Lloyd J, Kilpatrick CJ, Salzberg MR, Velakoulis D. Neuropsychiatric morbidity in focal epilepsy. British Journal of Psychiatry 2008;192(6):464‐9. [PUBMED: 18515901] - PubMed
-
- Alper K, Schwartz KA, Kolts RL, Khan A. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biological Psychiatry 2007;62(4):345‐54. [PUBMED: 17223086] - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual. 4th Edition. Washington DC: American Psychiatric Association, 2000.
-
- Anhoury S, Brown RJ, Krishnamoorthy ES, Trimble MR. Psychiatric outcome after temporal lobectomy: a predictive study. Epilepsia 2000;41(12):1608‐15. - PubMed
-
- Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. Journal of Neurochemistry 2007;100(4):857‐73. [PUBMED: 17212700] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

