Role of Aeromonas hydrophila flagella glycosylation in adhesion to Hep-2 cells, biofilm formation and immune stimulation
- PMID: 25464381
- PMCID: PMC4284686
- DOI: 10.3390/ijms151221935
Role of Aeromonas hydrophila flagella glycosylation in adhesion to Hep-2 cells, biofilm formation and immune stimulation
Abstract
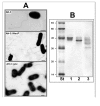
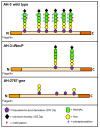
Polar flagellin proteins from Aeromonas hydrophila strain AH-3 (serotype O34) were found to be O-glycosylated with a heterogeneous heptasaccharide glycan. Two mutants with altered (light and strong) polar flagella glycosylation still able to produce flagella were previously obtained, as well as mutants lacking the O34-antigen lipopolysaccharide (LPS) but with unaltered polar flagella glycosylation. We compared these mutants, altogether with the wild type strain, in different studies to conclude that polar flagella glycosylation is extremely important for A. hydrophila adhesion to Hep-2 cells and biofilm formation. Furthermore, the polar flagella glycosylation is an important factor for the immune stimulation of IL-8 production via toll receptor 5 (TLR5).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

