Tumoral TP53 and/or CDKN2A alterations are not reliable prognostic biomarkers in patients with localized Ewing sarcoma: a report from the Children's Oncology Group
- PMID: 25464386
- PMCID: PMC4376595
- DOI: 10.1002/pbc.25340
Tumoral TP53 and/or CDKN2A alterations are not reliable prognostic biomarkers in patients with localized Ewing sarcoma: a report from the Children's Oncology Group
Abstract
Background: A growing collection of retrospective studies have suggested that TP53 mutations and/or CDKN2A deletions have prognostic significance in Ewing sarcoma. We sought to evaluate these variables in patients with localized disease treated prospectively on a single Children's Oncology Group protocol.
Procedure: Of the 568 patients enrolled on Children's Oncology Group protocol AEWS0031 (NCT00006734), 112 had tumor specimens of sufficient quality and quantity to allow for analysis of TP53 mutations status by DNA sequencing, and CDKN2A deletion by dual color fluorescent in situ hybridization.
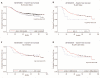
Results: Eight of 93 cases (8.6%) were found to have TP53 point mutations and 12 of 107 cases (11.2%) demonstrated homozygous CDKN2A deletion. Two cases were found to have an alteration in both genes. There was no significant difference in event-free survival of patients with TP53 mutations and/or CDKN2A deletions compared to patients with normal TP53/CDKN2A gene status, as demonstrated by log rank test (p = 0.58).
Conclusions: Although previous retrospective studies suggest their significance, TP53 mutation and/or CDKN2A deletion are not reliable prognostic biomarkers in localized Ewing sarcoma.
Keywords: Ewing sarcoma; biomarker; outcomes; prognosis.
© 2014 Wiley Periodicals, Inc.
Figures
Comment in
-
Ewing sarcoma: a tough road to clinically relevant biomarkers.Pediatr Blood Cancer. 2015 May;62(5):741-2. doi: 10.1002/pbc.25406. Epub 2015 Jan 13. Pediatr Blood Cancer. 2015. PMID: 25586427 No abstract available.
References
-
- Pizzo PA, Poplack DG. Principles and practice of pediatric oncology. Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia: 2011.
-
- Wang CC, Schulz MD. Ewing's sarcoma; a study of fifty cases treated at the Massachusetts General Hospital, 1930-1952 inclusive. N Engl J Med. 1953;248(14):571–576. - PubMed
-
- Dahlin DC, Coventry MB, Scanlon PW. Ewing's sarcoma. A critical analysis of 165 cases. The Journal of bone and joint surgery American volume. 1961;43-A:185–192. - PubMed
-
- Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(33):4148–4154. - PMC - PubMed
-
- Felgenhauer JL, Nieder ML, Krailo MD, et al. A pilot study of low-dose anti-angiogenic chemotherapy in combination with standard multiagent chemotherapy for patients with newly diagnosed metastatic Ewing sarcoma family of tumors: A Children's Oncology Group (COG) Phase II study NCT00061893. Pediatric blood & cancer. 2013;60(3):409–414. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

