Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation
- PMID: 25464848
- PMCID: PMC4268066
- DOI: 10.1016/j.celrep.2014.10.055
Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation
Abstract
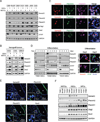
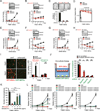
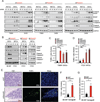
Different cancer cell compartments often communicate through soluble factors to facilitate tumor growth. Glioma stem cells (GSCs) are a subset of tumor cells that resist standard therapy to contribute to disease progression. How GSCs employ a distinct secretory program to communicate with and nurture each other over the nonstem tumor cell (NSTC) population is not well defined. Here, we show that GSCs preferentially secrete Sema3C and coordinately express PlexinA2/D1 receptors to activate Rac1/nuclear factor (NF)-κB signaling in an autocrine/paracrine loop to promote their own survival. Importantly, Sema3C is not expressed in neural progenitor cells (NPCs) or NSTCs. Disruption of Sema3C induced apoptosis of GSCs, but not NPCs or NSTCs, and suppressed tumor growth in orthotopic models of glioblastoma. Introduction of activated Rac1 rescued the Sema3C knockdown phenotype in vivo. Our study supports the targeting of Sema3C to break this GSC-specific autocrine/paracrine loop in order to improve glioblastoma treatment, potentially with a high therapeutic index.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. - PubMed
-
- Blanc V, Nariculam J, Munson P, Freeman A, Klocker H, Masters J, Williamson M. A role for class 3 semaphorins in prostate cancer. Prostate. 2011;71:649–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

