Stable luciferase expression does not alter immunologic or in vivo growth properties of GL261 murine glioma cells
- PMID: 25464980
- PMCID: PMC4258256
- DOI: 10.1186/s12967-014-0345-4
Stable luciferase expression does not alter immunologic or in vivo growth properties of GL261 murine glioma cells
Abstract
Background: GL261 cells are murine glioma cells that demonstrate proliferation, invasion, and angiogenesis when implanted in syngeneic C57BL/6 mice, providing a highly useful immunocompetent animal model of glioblastoma. Modification of tumor cells for luciferase expression enables non-invasive monitoring of orthotopic tumor growth, and has proven useful for studying glioblastoma response to novel therapeutics. However, tumor modification for luciferase has the potential for evoking host immune response against otherwise syngeneic tumor cells, thereby mitigating the tumor cells' value for tumor immunology and immunotherapy studies.
Methods: GL261 cells were infected with lentivirus containing a gene encoding firefly luciferase (GL261.luc). In vitro proliferation of parental (unmodified) GL261 and GL261.luc was measured on days 0, 1, 2, 4, and 7 following plating, and the expression of 82 mouse cytokines and chemokines were analyzed by RT-PCR array. Cell lines were also evaluated for differences in invasion and migration in modified Boyden chambers. GL261 and GL261.luc cells were then implanted intracranially in C57BL/6 mice, with GL261.luc tumor growth monitored by quantitative bioluminescence imaging, and all mice were followed for survival to compare relative malignancy of tumor cells.
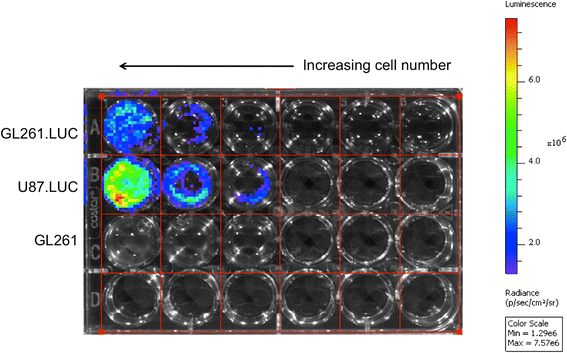
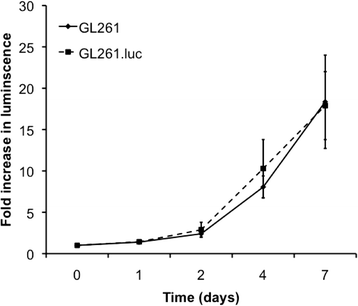
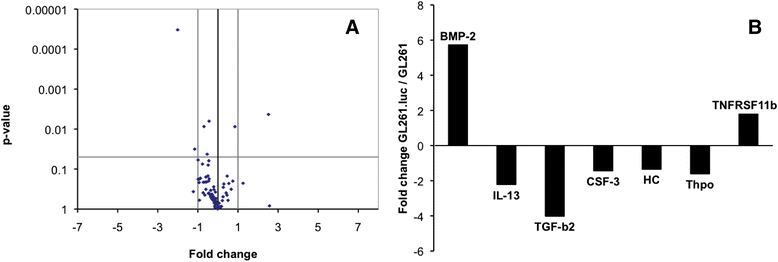
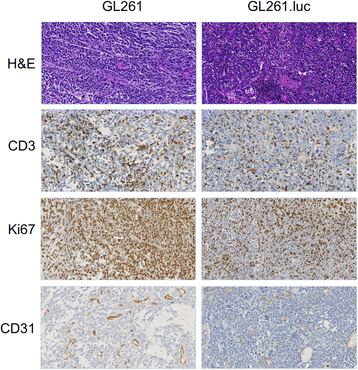
Results: No difference in proliferation was indicated for GL261 vs. GL261.luc cells (p>0.05). Of the 82 genes examined by RT-PCR array, seven (9%) exhibited statistically significant change after luciferase modification. Of these, only three changed by greater than 2-fold: BMP-2, IL-13, and TGF-β2. No difference in invasion (p=0.67) or migration (p=0.26) was evident between modified vs. unmodified cells. GL261.luc cell luminescence was detectable in the brains of C57BL/6 mice at day 5 post-implantation, and tumor bioluminescence increased exponentially to day 19. Median overall survival was 20.2 days versus 19.7 days for mice receiving implantation with GL261 and GL261.luc, respectively (p=0.62). Histopathologic analysis revealed no morphological difference between tumors, and immunohistochemical analysis showed no significant difference for staining of CD3, Ki67, or CD31 (p>0.05 for all).
Conclusions: Luciferase expression in GL261 murine glioma cells does not affect GL261 proliferation, invasion, cytokine expression, or in vivo growth. Luciferase modification increases their utility for studying tumor immunology and immunotherapeutic approaches for treating glioblastoma.
Figures



Similar articles
-
Bioluminescence Imaging of an Immunocompetent Animal Model for Glioblastoma.J Vis Exp. 2016 Jan 15;(107):e53287. doi: 10.3791/53287. J Vis Exp. 2016. PMID: 26863490 Free PMC article.
-
Intracranial implantation with subsequent 3D in vivo bioluminescent imaging of murine gliomas.J Vis Exp. 2011 Nov 6;(57):e3403. doi: 10.3791/3403. J Vis Exp. 2011. PMID: 22158303 Free PMC article.
-
Increasing glioma-associated monocytes leads to increased intratumoral and systemic myeloid-derived suppressor cells in a murine model.Neuro Oncol. 2015 Jul;17(7):978-91. doi: 10.1093/neuonc/nou343. Epub 2014 Dec 23. Neuro Oncol. 2015. PMID: 25537019 Free PMC article.
-
Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model.Cancer Immunol Immunother. 2011 Feb;60(2):153-60. doi: 10.1007/s00262-010-0946-6. Epub 2010 Dec 1. Cancer Immunol Immunother. 2011. PMID: 21120655 Free PMC article. Review.
-
Anti-PD-1 checkpoint blockade monotherapy in the orthotopic GL261 glioma model: the devil is in the detail.Neurooncol Adv. 2021 May 14;3(1):vdab066. doi: 10.1093/noajnl/vdab066. eCollection 2021 Jan-Dec. Neurooncol Adv. 2021. PMID: 34151268 Free PMC article. Review.
Cited by
-
Portable bioluminescent platform for in vivo monitoring of biological processes in non-transgenic animals.Nat Commun. 2021 May 11;12(1):2680. doi: 10.1038/s41467-021-22892-9. Nat Commun. 2021. PMID: 33976191 Free PMC article.
-
Mouse models of glioblastoma for the evaluation of novel therapeutic strategies.Neurooncol Adv. 2021 Jul 26;3(1):vdab100. doi: 10.1093/noajnl/vdab100. eCollection 2021 Jan-Dec. Neurooncol Adv. 2021. PMID: 34466804 Free PMC article. Review.
-
Probing the glioma micro-environment: analysis using biopsy in combination with ultra-fast cyclic immunolabeling.bioRxiv [Preprint]. 2024 Jun 17:2024.06.15.599078. doi: 10.1101/2024.06.15.599078. bioRxiv. 2024. Update in: Neoplasia. 2024 Nov;57:101051. doi: 10.1016/j.neo.2024.101051. PMID: 38948851 Free PMC article. Updated. Preprint.
-
An immunocompetent mouse model of human glioblastoma.Oncotarget. 2017 May 15;8(37):61072-61082. doi: 10.18632/oncotarget.17851. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 28977847 Free PMC article.
-
CRMP2 Phosphorylation Drives Glioblastoma Cell Proliferation.Mol Neurobiol. 2018 May;55(5):4403-4416. doi: 10.1007/s12035-017-0653-9. Epub 2017 Jun 28. Mol Neurobiol. 2018. PMID: 28660485 Free PMC article.
References
-
- Ausman JI, Shapiro WR, Rall DP. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970;30:2394–2400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

