The dicer-like1 homolog fuzzy tassel is required for the regulation of meristem determinacy in the inflorescence and vegetative growth in maize
- PMID: 25465405
- PMCID: PMC4311206
- DOI: 10.1105/tpc.114.132670
The dicer-like1 homolog fuzzy tassel is required for the regulation of meristem determinacy in the inflorescence and vegetative growth in maize
Abstract
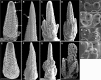
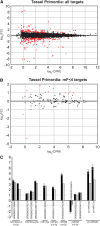
Plant architecture is determined by meristems that initiate leaves during vegetative development and flowers during reproductive development. Maize (Zea mays) inflorescences are patterned by a series of branching events, culminating in floral meristems that produce sexual organs. The maize fuzzy tassel (fzt) mutant has striking inflorescence defects with indeterminate meristems, fasciation, and alterations in sex determination. fzt plants have dramatically reduced plant height and shorter, narrower leaves with leaf polarity and phase change defects. We positionally cloned fzt and discovered that it contains a mutation in a dicer-like1 homolog, a key enzyme required for microRNA (miRNA) biogenesis. miRNAs are small noncoding RNAs that reduce target mRNA levels and are key regulators of plant development and physiology. Small RNA sequencing analysis showed that most miRNAs are moderately reduced in fzt plants and a few miRNAs are dramatically reduced. Some aspects of the fzt phenotype can be explained by reduced levels of known miRNAs, including miRNAs that influence meristem determinacy, phase change, and leaf polarity. miRNAs responsible for other aspects of the fzt phenotype are unknown and likely to be those miRNAs most severely reduced in fzt mutants. The fzt mutation provides a tool to link specific miRNAs and targets to discrete phenotypes and developmental roles.
© 2014 American Society of Plant Biologists. All rights reserved.
Figures



Comment in
-
A world beyond Arabidopsis: updates on small RNAs in plant development.Plant Cell. 2014 Dec;26(12):4564. doi: 10.1105/tpc.114.134635. Epub 2014 Dec 2. Plant Cell. 2014. PMID: 25465406 Free PMC article. No abstract available.
References
-
- Aichinger E., Kornet N., Friedrich T., Laux T. (2012). Plant stem cell niches. Annu. Rev. Plant Biol. 63: 615–636. - PubMed
-
- Ambrose B.A., Lerner D.R., Ciceri P., Padilla C.M., Yanofsky M.F., Schmidt R.J. (2000). Molecular and genetic analyses of the silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Mol. Cell 5: 569–579. - PubMed
-
- Bartel D.P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116: 281–297. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

