The role of TRPMLs in endolysosomal trafficking and function
- PMID: 25465891
- PMCID: PMC4412768
- DOI: 10.1016/j.ceca.2014.10.008
The role of TRPMLs in endolysosomal trafficking and function
Abstract
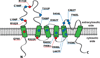
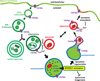
Members of the Transient Receptor Potential-Mucolipin (TRPML) constitute a family of evolutionarily conserved cation channels that function predominantly in endolysosomal vesicles. Whereas loss-of-function mutations in human TRPML1 were first identified as being causative for the lysosomal storage disease, Mucolipidosis type IV, most mammals also express two other TRPML isoforms called TRPML2 and TRPML3. All three mammalian TRPMLs as well as TRPML related genes in other species including Caenorhabditis elegans and Drosophila exhibit overlapping functional and biophysical properties. The functions of TRPML proteins include roles in vesicular trafficking and biogenesis, maintenance of neuronal development, function, and viability, and regulation of intracellular and organellar ionic homeostasis. Biophysically, TRPML channels are non-selective cation channels exhibiting variable permeability to a host of cations including Na(+), Ca(2+), Fe(2+), and Zn(2+), and are activated by a phosphoinositide species, PI(3,5)P2, that is mostly found in endolysosomal membranes. Here, we review the functional and biophysical properties of these enigmatic cation channels, which represent the most ancient and archetypical TRP channels.
Keywords: Autophagy; Ca(2+); Endolysosomal; Endosomal; Late-endosomal; Lysosomal; ML4; MLIV; Mucolipidosis type IV; Mucolipins; TRP channels; TRPML.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Berman ER, et al. Congenital corneal clouding with abnormal systemic storage bodies: a new variant of mucolipidosis. J Pediatr. 1974;84(4):519–526. - PubMed
-
- Livni N, Legum C. Ultrastructure of cultured fibroblasts in mucolipidosis type IV. Exp Cell Biol. 1976;44(1):1–11. - PubMed
-
- Merin S, et al. The cornea in mucolipidosis IV. J Pediatr Ophthalmol. 1976;13(5):289–295. - PubMed
-
- Tellez-Nagel I, et al. Mucolipidosis IV. Clinical, ultrastructural, histochemical, and chemical studies of a case, including a brain biopsy. Arch Neurol. 1976;33(12):828–835. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

