Loss-of-function mutations in WDR73 are responsible for microcephaly and steroid-resistant nephrotic syndrome: Galloway-Mowat syndrome
- PMID: 25466283
- PMCID: PMC4259970
- DOI: 10.1016/j.ajhg.2014.10.011
Loss-of-function mutations in WDR73 are responsible for microcephaly and steroid-resistant nephrotic syndrome: Galloway-Mowat syndrome
Abstract
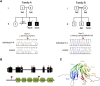
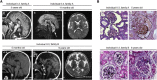
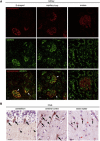
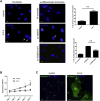
Galloway-Mowat syndrome is a rare autosomal-recessive condition characterized by nephrotic syndrome associated with microcephaly and neurological impairment. Through a combination of autozygosity mapping and whole-exome sequencing, we identified WDR73 as a gene in which mutations cause Galloway-Mowat syndrome in two unrelated families. WDR73 encodes a WD40-repeat-containing protein of unknown function. Here, we show that WDR73 was present in the brain and kidney and was located diffusely in the cytoplasm during interphase but relocalized to spindle poles and astral microtubules during mitosis. Fibroblasts from one affected child and WDR73-depleted podocytes displayed abnormal nuclear morphology, low cell viability, and alterations of the microtubule network. These data suggest that WDR73 plays a crucial role in the maintenance of cell architecture and cell survival. Altogether, WDR73 mutations cause Galloway-Mowat syndrome in a particular subset of individuals presenting with late-onset nephrotic syndrome, postnatal microcephaly, severe intellectual disability, and homogenous brain MRI features. WDR73 is another example of a gene involved in a disease affecting both the kidney glomerulus and the CNS.
Copyright © 2014 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Pezzella M., Yeghiazaryan N.S., Veggiotti P., Bettinelli A., Giudizioso G., Zara F., Striano P., Minetti C. Galloway-Mowat syndrome: an early-onset progressive encephalopathy with intractable epilepsy associated to renal impairment. Two novel cases and review of literature. Seizure. 2010;19:132–135. - PubMed
-
- Ekstrand J.J., Friedman A.L., Stafstrom C.E. Galloway-Mowat syndrome: neurologic features in two sibling pairs. Pediatr. Neurol. 2012;47:129–132. - PubMed
-
- Meyers K.E., Kaplan P., Kaplan B.S. Nephrotic syndrome, microcephaly, and developmental delay: three separate syndromes. Am. J. Med. Genet. 1999;82:257–260. - PubMed
-
- Hazza I., Najada A.H. Late-onset nephrotic syndrome in galloway-mowat syndrome: a case report. Saudi J. Kidney Dis. Transpl. 1999;10:171–174. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

