Increased risk of everolimus-associated acute kidney injury in cancer patients with impaired kidney function
- PMID: 25466872
- PMCID: PMC4265483
- DOI: 10.1186/1471-2407-14-906
Increased risk of everolimus-associated acute kidney injury in cancer patients with impaired kidney function
Abstract
Background: Everolimus was recently introduced as a second-line treatment for renal cell carcinoma (RCC) and many other cancers. Several prospective studies have shown that serum creatinine levels are increased in a significant proportion of patients receiving everolimus. However, data on the occurrence of acute kidney injury (AKI) during everolimus treatment in clinical practice are sparse. Here, we report the incidence, risk factors, and clinical significance of AKI associated with everolimus treatment in patients with cancer.
Methods: We analyzed patients who received everolimus for more than 4 weeks as an anticancer therapy. AKI was defined as increase in creatinine levels from baseline levels greater than 1.5-fold.
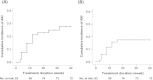
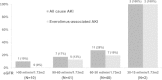
Results: The majority of the 110 patients enrolled in this analysis had RCC (N=93, 84.5%). AKI developed in 21 (23%) RCC patients; none of the patients (N=17) with other cancers had AKI. Fourteen of 21 cases were considered to be everolimus-associated AKI, in which there were no other nephrotoxic insults other than everolimus at the onset of AKI. The incidence of AKI increased progressively as baseline estimated glomerular filtration rate (eGFR) decreased (10% in subjects with eGFR >90 mL/min/1.73 m2, 17% in subjects with eGFR 60-90 mL/min/1.73 m2, 28% in subjects with eGFR 30-60 mL/min/1.73 m2, and 100% in subjects with eGFR 15-30 mL/min/1.73 m2; P=0.029 for trend). Baseline eGFR was an independent risk factor for the development of everolimus-associated AKI (hazard ratio per 10 mL/min/1.73 m2 increase, 0.70; 95% confidential interval, 049-1.00; P=0.047). Nine of 14 patients with everolimus-associated AKI continued receiving the drug at a reduced dose or after a short-term off period. Administration of the drug was discontinued in four of 14 patients because of progression of an underlying malignancy. Only one patient stopped taking the drug because of AKI.
Conclusions: This paper suggests that AKI is a common adverse effect of everolimus treatment, especially in subjects with impaired renal function. However, the occurrence of AKI did not require the discontinuation of the drug, and the treatment decision should be made via a multidisciplinary approach, including the assessment of the oncological benefits of everolimus and other therapeutic options.
Figures
Similar articles
-
Understanding the Risk Factors and Long-Term Consequences of Cisplatin-Associated Acute Kidney Injury: An Observational Cohort Study.PLoS One. 2015 Nov 10;10(11):e0142225. doi: 10.1371/journal.pone.0142225. eCollection 2015. PLoS One. 2015. PMID: 26556481 Free PMC article.
-
Everolimus in acute kidney injury in a patient with breast cancer: a case report.J Med Case Rep. 2014 Nov 25;8:386. doi: 10.1186/1752-1947-8-386. J Med Case Rep. 2014. PMID: 25420955 Free PMC article.
-
Immune-related adverse events and kidney function decline in patients with genitourinary cancers treated with immune checkpoint inhibitors.Eur J Cancer. 2021 Nov;157:50-58. doi: 10.1016/j.ejca.2021.07.031. Epub 2021 Sep 2. Eur J Cancer. 2021. PMID: 34482189
-
Everolimus in the treatment of renal cell carcinoma and neuroendocrine tumors.Adv Ther. 2010 Aug;27(8):495-511. doi: 10.1007/s12325-010-0045-2. Epub 2010 Jul 8. Adv Ther. 2010. PMID: 20623346 Review.
-
Everolimus for the treatment of advanced renal cell carcinoma.Expert Opin Pharmacother. 2011 May;12(7):1143-55. doi: 10.1517/14656566.2011.571382. Epub 2011 Apr 7. Expert Opin Pharmacother. 2011. PMID: 21470068 Review.
Cited by
-
Renal toxicity of targeted therapies for renal cell carcinoma in patients with normal and impaired kidney function.Cancer Chemother Pharmacol. 2021 Jun;87(6):723-742. doi: 10.1007/s00280-021-04260-y. Epub 2021 Mar 25. Cancer Chemother Pharmacol. 2021. PMID: 33768301 Free PMC article. Review.
-
Complex Monitoring of Biochemical and Radionuclide Parameters in Patients with Metastatic Renal Cell Carcinoma during Immunotherapy.Int J Nephrol. 2017;2017:8549502. doi: 10.1155/2017/8549502. Epub 2017 Oct 22. Int J Nephrol. 2017. PMID: 29201463 Free PMC article.
-
Effect of everolimus treatment for regrown renal angiomyolipoma associated with tuberous sclerosis complex after transcatheter arterial embolization.Int J Clin Oncol. 2018 Dec;23(6):1134-1139. doi: 10.1007/s10147-018-1325-0. Epub 2018 Aug 1. Int J Clin Oncol. 2018. PMID: 30069798
-
Anticancer Drug-Induced Acute Kidney Injury.Kidney Int Rep. 2017 Feb 16;2(4):504-514. doi: 10.1016/j.ekir.2017.02.008. eCollection 2017 Jul. Kidney Int Rep. 2017. PMID: 29318217 Free PMC article. Review.
-
Efficacy and safety of sorafenib for advanced renal cell carcinoma: real-world data of patients with renal impairment.Oncotarget. 2018 Apr 10;9(27):19406-19414. doi: 10.18632/oncotarget.24779. eCollection 2018 Apr 10. Oncotarget. 2018. PMID: 29721212 Free PMC article.
References
-
- Pascual J. Everolimus in clinical practice--renal transplantation. Nephrol Dial Transplant. 2006;21 Suppl 3:iii18–iii23. - PubMed
-
- Mjornstedt L, Sorensen SS, von Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, Andersson H, Gustafsson B, Undset LH, Fagertun H, Solbu D, Holdaas H. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. Am J Transplant. 2012;12:2744–2753. doi: 10.1111/j.1600-6143.2012.04162.x. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/906/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

