Quantitative separation of arterial and venous cerebral blood volume increases during voluntary locomotion
- PMID: 25467301
- PMCID: PMC4330110
- DOI: 10.1016/j.neuroimage.2014.10.030
Quantitative separation of arterial and venous cerebral blood volume increases during voluntary locomotion
Abstract
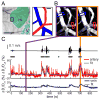
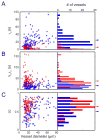
Voluntary locomotion is accompanied by large increases in cortical activity and localized increases in cerebral blood volume (CBV). We sought to quantitatively determine the spatial and temporal dynamics of voluntary locomotion-evoked cerebral hemodynamic changes. We measured single vessel dilations using two-photon microscopy and cortex-wide changes in CBV-related signal using intrinsic optical signal (IOS) imaging in head-fixed mice freely locomoting on a spherical treadmill. During bouts of locomotion, arteries dilated rapidly, while veins distended slightly and recovered slowly. The dynamics of diameter changes of both vessel types could be captured using a simple linear convolution model. Using these single vessel measurements, we developed a novel analysis approach to separate out spatially and temporally distinct arterial and venous components of the location-specific hemodynamic response functions (HRF) for IOS. The HRF of each pixel of was well fit by a sum of a fast arterial and a slow venous component. The HRFs of pixels in the limb representations of somatosensory cortex had a large arterial contribution, while in the frontal cortex the arterial contribution to the HRF was negligible. The venous contribution was much less localized, and was substantial in the frontal cortex. The spatial pattern and amplitude of these HRFs in response to locomotion in the cortex were robust across imaging sessions. Separating the more localized arterial component from the diffuse venous signals will be useful for dealing with the dynamic signals generated by naturalistic stimuli.
Keywords: Exercise; Hemodynamics; Intrinsic optical imaging; Linear model; Two-photon microscopy.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


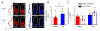


References
-
- Aquino KM, Robinson PA, Schira MM, Breakspear M. Deconvolution of neural dynamics from fMRI data using a spatiotemporal hemodynamic response function. NeuroImage. 2014;94:203–215. - PubMed
-
- Arieli A, Grinvald A, Slovin H. Dural substitute for long-term imaging of cortical activity in behaving monkeys and its clinical implications. J Neurosci Methods. 2002;114:119–133. - PubMed
-
- Barrett MJP, Tawhai MH, Suresh V. Arteries dominate volume changes during brief functional hyperemia: Evidence from mathematical modelling. NeuroImage. 2012;62:482–492. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

