Identification of spinal circuits transmitting and gating mechanical pain
- PMID: 25467445
- PMCID: PMC4258511
- DOI: 10.1016/j.cell.2014.11.003
Identification of spinal circuits transmitting and gating mechanical pain
Abstract
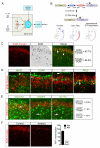
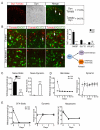
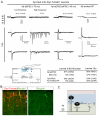
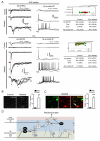
Pain information processing in the spinal cord has been postulated to rely on nociceptive transmission (T) neurons receiving inputs from nociceptors and Aβ mechanoreceptors, with Aβ inputs gated through feed-forward activation of spinal inhibitory neurons (INs). Here, we used intersectional genetic manipulations to identify these critical components of pain transduction. Marking and ablating six populations of spinal excitatory and inhibitory neurons, coupled with behavioral and electrophysiological analysis, showed that excitatory neurons expressing somatostatin (SOM) include T-type cells, whose ablation causes loss of mechanical pain. Inhibitory neurons marked by the expression of dynorphin (Dyn) represent INs, which are necessary to gate Aβ fibers from activating SOM(+) neurons to evoke pain. Therefore, peripheral mechanical nociceptors and Aβ mechanoreceptors, together with spinal SOM(+) excitatory and Dyn(+) inhibitory neurons, form a microcircuit that transmits and gates mechanical pain. PAPERCLIP:
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



References
-
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. - PubMed
-
- Bennett GJ, Abdelmoumene M, Hayashi H, Dubner R. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. J Comp Neurol. 1980;194:809–827. - PubMed
-
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. - PubMed
-
- Bini G, Cruccu G, Hagbarth KE, Schady W, Torebjörk E. Analgesic effect of vibration and cooling on pain induced by intraneural electrical stimulation. Pain. 1984;18:239–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK089044/DK/NIDDK NIH HHS/United States
- R01 NS 080586/NS/NINDS NIH HHS/United States
- R01 DK096010/DK/NIDDK NIH HHS/United States
- R37 DK053477/DK/NIDDK NIH HHS/United States
- R01NS086372/NS/NINDS NIH HHS/United States
- R01 DK071051/DK/NIDDK NIH HHS/United States
- R01 NS086372/NS/NINDS NIH HHS/United States
- P01 NS072040/NS/NINDS NIH HHS/United States
- P30 DK046200/DK/NIDDK NIH HHS/United States
- P01 NS072031/NS/NINDS NIH HHS/United States
- R01 NS080586/NS/NINDS NIH HHS/United States
- P01 NS047572/NS/NINDS NIH HHS/United States
- F32 DK089710/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- R01 DK075632/DK/NIDDK NIH HHS/United States
- R01 AR063772/AR/NIAMS NIH HHS/United States
- R21 AR064445/AR/NIAMS NIH HHS/United States
- NS047710/NS/NINDS NIH HHS/United States
- R01 NS047710/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

